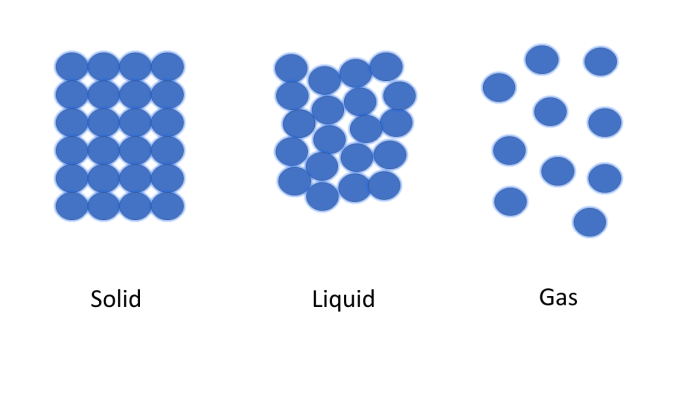
The three states of matter are solid, liquid and gas. In solids, particles are held in a fixed pattern by strong bonds, but they vibrate. In liquids, the bonds between particles are weaker than in solids; the particles are still close to one another but are randomly arranged and free to move. In gases, particles are spread out widely and move faster than in solids and liquids.
The following diagram shows the arrangement of particles in the three states of matter:
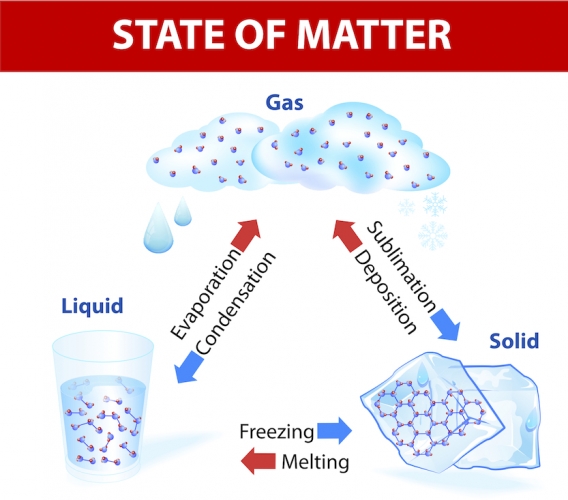
When a solid is heated and reaches its melting point, it melts into a liquid.
When a liquid reaches its boiling point, it evaporates into a gas.
When you cool down a gas, you can condense it back into a liquid and a liquid turns into a solid when it reaches its freezing point.
The freezing and melting points of a substance are always the same temperatures.
Sublimation is when a solid turns straight into a gas.
Pure water becomes ice at 0oC and it boils at 100oC.
As the bonds that hold solid particles together are very strong, heat energy is needed to break them, and the particles start moving more freely when the substance becomes a liquid. When a solid turns back into a liquid, this energy is transferred to the surroundings, as it is no longer needed.
Similarly, a liquid needs heat energy to change it to a gas. An example of this is when we sweat. The water in the sweat uses heat from our bodies to evaporate and this has the effect of cooling us down. A dog cools itself by sticking its tongue out and panting. The saliva on its tongue evaporates taking heat from the tongue as it does so.

There's lots more to learn about this topic but let's have a go at the questions now.

