There are two types of changes in the world around us: physical and chemical.
Physical changes
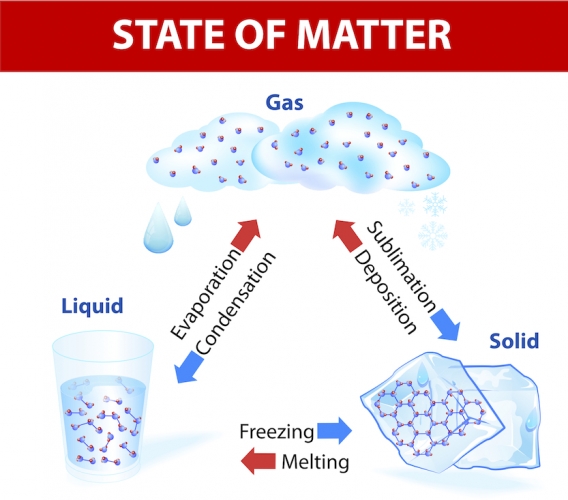
If you let ice melt, it turns into water. There has been a change of state from solid to liquid, but it is still the same substance. It is easy to freeze water and make it ice again. You can even boil it and make it steam. It is still the same substance and no new substances have formed. These changes are reversible, which means the substance can turn back to its original state. These are called physical changes.
In physical changes the atoms remain the same and no chemical bonds change. For example, in water, two hydrogen atoms are still bonded to an oxygen atom, regardless of whether you have ice, water or steam.
Chemical changes
Chemical changes take place during chemical reactions.
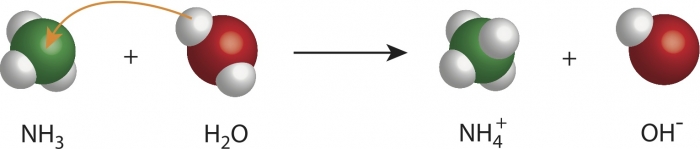
In the image above, rearrangement of atoms is clearly shown for the production of ammonia: you can see one hydrogen atom from the water molecule joining the ammonia molecule, which now becomes NH4 (ammonium). The atoms remain the same, but their positions are rearranged, which means they bond with different atoms after the chemical change.
Chemical changes are permanent changes and cannot be reversed; they are irreversible. When a piece of wood burns, you cannot reverse the reaction to get the wood back. New substances have formed.

