Before we look at displacement reactions, it is important to review the reactivity series.
The reactivity series lists metals in order of reactivity from the most reactive to the least reactive.
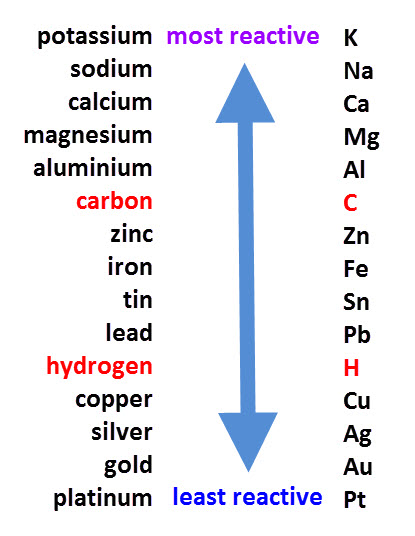
When looking at displacement reactions, it is important to refer to the reactivity series because a displacement reaction is one in which a metal displaces a less reactive metal from a compound.
Look at the pictures below. The man on the left is weaker and unfit in comparison to the man on the right, who is strong and muscular. If they were lifting heavy weights, who would win?


The man on the right would win - he is after all a lot stronger with big muscles! The stronger person picks up the heavier weight and beats the weaker person. We can use this idea to model displacement. The 'stronger' or more reactive element takes the place of the 'weaker' or less reactive element.
This is better illustrated in an equation:
magnesium + copper sulfate ![]() magnesium sulfate + copper
magnesium sulfate + copper
The magnesium is the strong man and the copper is the weak man, because magnesium is higher up the reactivity series than copper. The copper is displaced from the compound by the magnesium because the magnesium is more reactive.
In the lab, this reaction would result in a colour change. Copper sulfate is blue in colour and the copper metal formed is brown in colour, so the solution would turn from blue to colourless (magnesium sulfate solution) and the silvery metal magnesium would gain a coating of brown metal (copper). Colour changes, like these, indicate that a reaction is going on.
 |
 |
| copper sulfate | copper metal |
The reactivity series (see above) can be used to predict displacement reactions.
For example:
Zinc will react with iron nitrate because zinc is more reactive than iron, but zinc will not react with magnesium nitrate because zinc is less reactive than magnesium.
zinc + iron nitrate ![]() zinc nitrate + iron
zinc nitrate + iron
zinc + magnesium nitrate ![]() NO REACTION
NO REACTION
Displacement reactions occur in solution when the more reactive metal displaces (takes the place of) the less reactive metal and so forms a soluble salt.
In the example above, the zinc displaces the iron from the solution. That means that it takes its place as the 'partner' of the nitrate ion and becomes the salt zinc nitrate. Meanwhile, the iron is all on its own and so is deposited as the metal iron on the bottom of the beaker.
Probably best to read this all through again, thinking about what's happening in terms of reactivity, and then try out the questions and see how it goes, OK?
If you need to check back to the introduction at any point, just click on the red help button on the side of the screen.

