Cooking food is a chemical reaction that takes place everyday in most households. A chemical reaction, such as cooking an egg is an irreversible change, because we cannot bring the egg back to its original raw state. A physical change is one that can be reversed; for example, we can boil water and then let it cool again. This is called a reversible change.
Chemical reactions are irreversible changes. Some of them take place when a substance decomposes (breaks down) when heated. An example of this is the thermal decomposition of sodium hydrogencarbonate, comonly known as baking powder. We can show this reaction using a chemical equation:
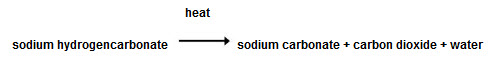
We use baking powder in baking, because when it is heated it produces carbon dioxide, a gas that gets trapped in the dough and makes it rise.
Burning fuels (oil, wood, gas and even paper) is also a chemical reaction:
fuel + oxygen ![]() carbon dioxide + water
carbon dioxide + water
The scientific name for burning is combustion.
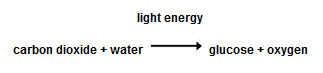
Some reactions are exothermic, which means they release heat. An example of this is combustion. However, some reactions need energy to take place and these are called endothermic. An example of endothermic reactions is photosynthesis in plants, where energy from the Sun is taken in.
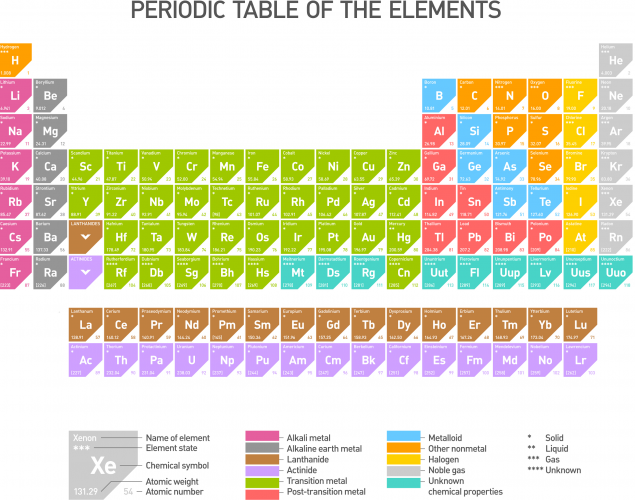
Group 1 elements of the Periodic table (shown below, click on it to see a larger version) react with water to produce hydroxides, which are alkalis:
lithium + water ![]() lithium hydroxide + hydrogen
lithium hydroxide + hydrogen
Group 7 elements can react with Group 1 elements in the following way:
sodium + chlorine ![]() sodium chloride
sodium chloride
Chemical reactions occur because elements either share or give/take electrons to/from each other. The bonds between atoms weaken and break and that's how new substances are formed. Elements or compounds before the arrow are called reactants, whereas after the arrow are called products.