Acids have a reputation for being nasty chemicals that burn through floors and metal structures with great ease. But did you realise that vinegar is an acid? This activity tackles some of the myths associated with acids and their chemical opposites - alkalis. Many of the products you find at home are acidic or alkaline.
Having vinegar on your chips is not dangerous, but drinking a lab acid like hydrochloric acid could be fatal. This is because hydrochloric acid is a much stronger acid than vinegar
.
The strength of an acid or alkali can be measured using the pH scale.
pH Scale
The pH scale is a measure of how strong or weak an acid or alkali is. It is a continuous scale from below 0 to above 14. The colour chart below shows the colour that universal indicator turns when it’s mixed with solutions of different pHs:
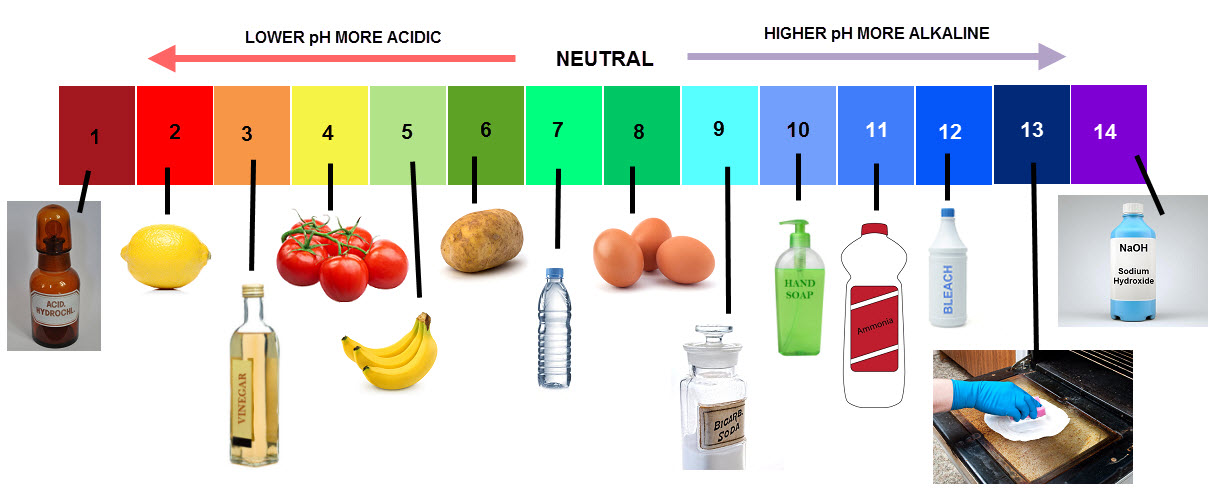
Neutral solutions such as water have a pH of 7.
Acids have a pH of less than 7. The lower the pH number of an acid the stronger the acid is. So an acid like hydrochloric acid with a pH of 1 is a lot stronger than vinegar with a pH of 3.
Alkalis have a pH of greater than 7. The higher the pH number of an alkali the stronger it is. So an alkali like sodium hydroxide with a pH of 14 is a lot stronger than baking soda with a pH of 9.
Let's make a start on these questions!

