Plants are valuable sources of chemical substances, which have many uses. Vegetable oils are an example of this. Apart from being used as oils, vegetable oils are the first material for making other food products, like margarine. They can also be used as fuels; for example, biodiesel.
Seeds, nuts and some fruit are squeezed in order to get the oil out; for example, olive oil. Some oils are a bit harder to obtain and need more processing. Vegetable oil molecules consist of glycerol and fatty acids.
Vegetable oils have a higher boiling point than water, so food is cooked differently when it is submerged in oils. They give food a different taste and, if food is cooked in them, it provides more energy. However, too much fried food is fattening and not healthy.
Fatty acids in vegetable oils can be saturated or unsaturated. The latter are healthier and better for our diets.
- Saturated fatty acids have a single bond between their carbon atoms and they are solid at room temperature. Unsaturated fatty acids have double bonds between their carbon atoms and are liquid in room temperature.
- Unsaturated fatty acids can be monounsaturated (with only one double bond) and polyunsaturated (with multiple double bonds). Unsaturated fats can be 'hardened' when reacting with hydrogen gas during a process called hydrogenation. This process converts the double bonds into single bonds.
You may already know that water and oil do not mix. A detergent helps a great deal to wash plates, because it enables oil to mix with water and be removed. Detergents are examples of emulsifiers; substances that help oil and water mix. Emulsions are mixtures of oil and water, which are used in cosmetics, paints and salad dressings. However, an emulsifier is needed to stabilise emulsions. For example, egg yolk contains a natural emulsifier, which helps bind oil and vinegar in mayonnaise.
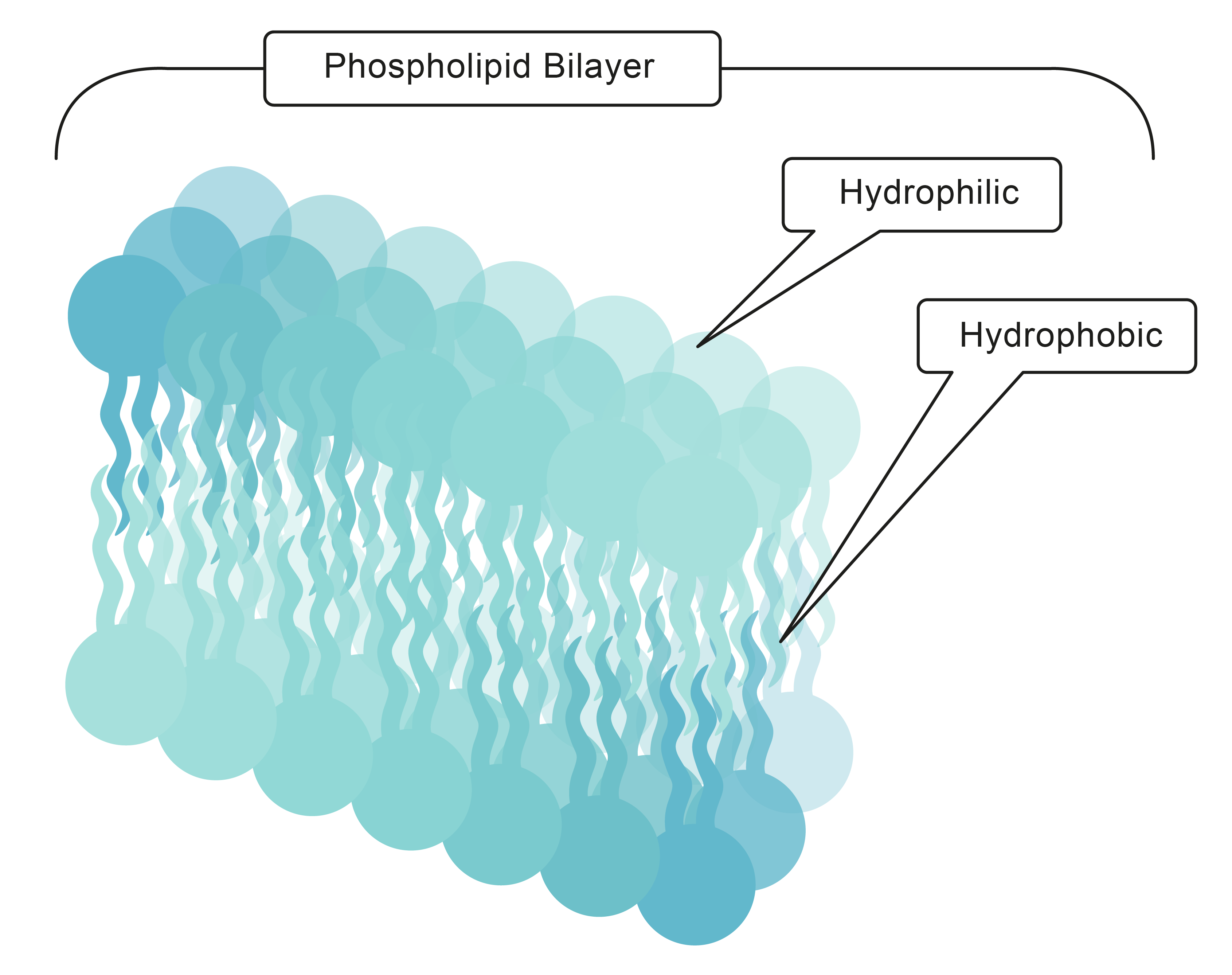
Emulsifier molecules have a hydrophobic tail (water-hating), which dissolves in the oil and a hydrophilic head (water-loving), which dissolves in the water. Have a look at the diagram below, which shows the molecule of an emulsifier. They both dissolve in their 'favourite' medium, which binds them together.