What would happen if you were to push a pin on its pointy end? You would hurt yourself, but why?
The pin would push on your finger so much that it would break the bonds between the cells and become physically inserted into your finger.
What would happen if you did the same, but this time with the flat end?
The pin would go into the wall, this time breaking the bonds of the atoms in the wall and making a nice snug home for itself.

Assuming you applied the same force in both cases, the end result would be different because of the difference in pressure applied to your thumb.
In the first case, the force is concentrated on a very small area, so the pressure is very high. In the second, the force is spread out over a larger area, so the pressure is lower.
The formula used to calculate pressure is:
Pressure = force/area
which means
Pressure = force ÷ area
When the force is in Newtons (N) and the area is m2, the unit for pressure is Pascal (Pa) or N/m2
.jpg)
Let's learn about gas pressure next.
Did you know that the air around you is an example of a fluid? Liquids and gases are both fluids because they have no fixed shape.
You might remember that in a gas, particles are spread far apart, and move in random directions with random speeds.
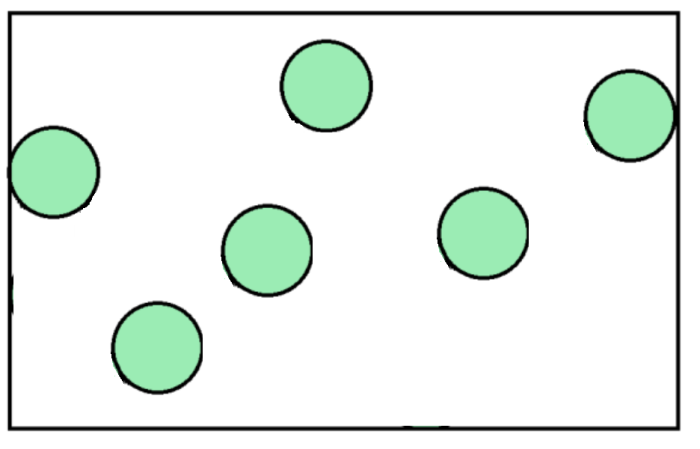
Use your imagination to visualise how gas particles collide into the walls of the containers in the diagram. Each time they hit the walls of the container, they push them a little bit. This is where our force comes from in the equation: pressure = force/area.
The area comes from the internal area of whatever container the gas is inside.
Gas pressure explains how balloons work. When we blow into a balloon, we add air. The air inside the balloon collides with the internal walls, providing a pressure. If the air pressure inside the balloon is greater than the air pressure outside the balloon, the balloon stretches and gets bigger!
Now let's try some practice questions on gas pressure.