You need to know the arrangements of particles in solids, liquids, and gases.
You also need to be able to explain what happens for each change of state. A flow diagram summarising each change is shown below.
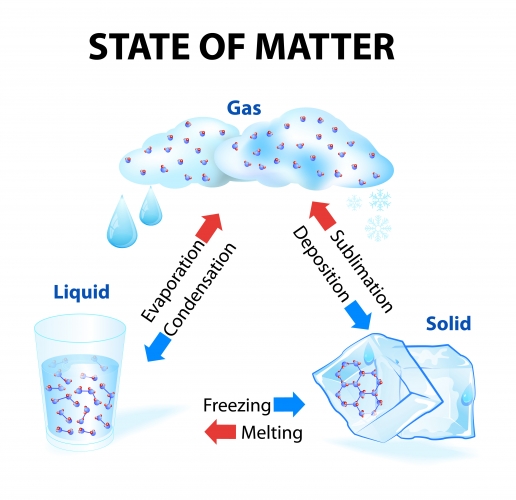
Any red arrows represent changes that involve an increase in temperature (i.e. melting, evaporation, and sublimation need you to heat something up). When these types of change occur, the particles gain kinetic energy, so they move faster. The forces between the particles also become weaker as the particles are able to spread further apart. In doing so, the substance becomes less dense.
The blue arrows represent changes of state that involve a decrease in temperature (i.e. condensation, freezing, and deposition need you to cool the substance down). The particles now behave in the opposite way: they lose kinetic energy and move slower. The forces between the particles become stronger, so they become closer together, making the substance more dense.
Changes of state are reversible. For example, if you melt a solid into a liquid, it is possible to turn it back into a solid if you cool it back down to the right temperature. The atoms that make the substance do not turn into a different chemical element or compound, and so we describe a change of state as a physical change, rather than a chemical change.
The ice-water anomaly

An 'anomaly' is an unexpected result that does not fit a pattern. The change of state between ice and water does not fit the behaviour of particles that we have just seen. When water turns to ice, it is a change of state from liquid to solid, but the particles move further apart (instead of closer together), and therefore the solid becomes less dense than the liquid (instead of more dense).
This unusual arrangement of particles in ice means that it is able to float on water.
For other elements or compounds, freezing them from a liquid to a solid would make them more dense and they would sink if they were placed into a container of their liquid form.
Right, let's have a go at some questions now.








