The diagram below shows the molecular models of various elements and compounds.
Look for the oxygen model at the bottom. It contains only oxygen atoms - two of them make an oxygen molecule.
A substance that is made of only one type of molecule is a pure substance. In the case of oxygen, there is only one type of atom, so this is actually a pure element.
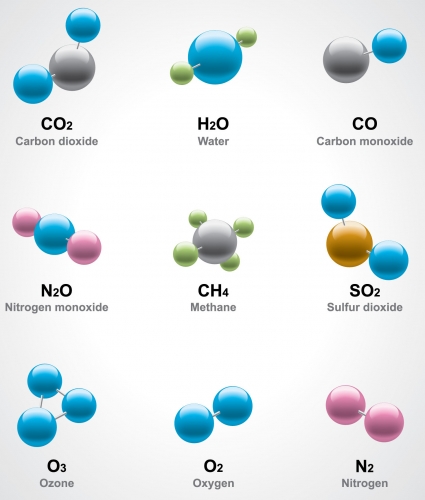
Look for the molecule of water at the top. It is made of oxygen and hydrogen atoms that have bonded together, so it is a compound. A substance made only of water molecules is pure water.
Seawater, on the other hand, is not a pure substance - it contains salt molecules dissolved in it, sand and seaweed. Seawater is a mixture. This means the water and salt molecules have mixed with each other, but they have not reacted (as in compounds), so they can easily be separated if we let the water evaporate. Furthermore, we are not usually able to see the sand in seawater, but if it settles a bit, we can see the sand at the bottom of the container. This is because sand does not dissolve (it's insoluble) in water.
A pure substance in chemistry is different to a pure substance in everyday life. For example, orange juice may be advertised as pure, but for a chemist, orange juice is a mixture of water, sugar, citric acid, vitamin C and other nutrients.
In order to identify a pure substance you need to look at how many different elements and/or compounds it is made of. If it only contains one type of element, or one type of compound, it is a pure substance.
Think you can recognise a pure substance or a mixture? Great! Let's try some questions.









