Everything around us exists in one of the three states of matter. Things can be solid, liquid or gas!

Take a look around you now. Can you spot any things that would be classed as solids? Your chair, your desk, a pen..... maybe you can see a cup or a plastic toy. All solids! We can tell they're solids because they can be held in your hand (if they're not too heavy!) as they have a fixed shape.

Liquids on the other hand, cannot be held in your hand - unless they are in a container, like a bottle or a cup. The milk you pour on your cereal, the juice you drink when thirsty, the rain that falls when it's a wet and grey day - all liquids. We can tell they are liquids because they can flow, or be easily poured. They can also change shape depending on what container they are in. Impressive hey!

Gases are more tricky to spot. This is because they are often colourless, and spread out all around us. The air we breathe is a gas. Car exhaust fumes can often be seen as a white or grey smoke - another gas. The helium used to inflate the balloons at a recent party you went to - gas again!

The reason why solids liquids and gases have quite different properties is because of the way their particles are arranged.
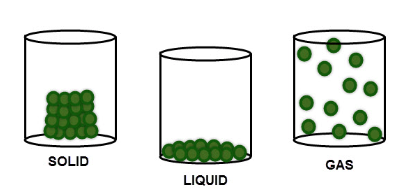
In a solid, the tiny particles that make up the solid are very close together in a neat regular arrangement, all touching each other and moving only by vibrating on the spot. They cannot move much at all, which explains why solids have a fixed shape.
The particles in a liquid are arranged in a more random way. They are close together, most of them touching each other, but there are some small gaps. So the particles can move around a little, over each other, allowing liquids to flow and be poured. This is the reason why liquids take the shape of the container they are in.
The particles in a gas are spaced very far apart, move around very fast and they are randomly arranged. Because of this, gases can be easily compressed, or squashed - they have no fixed shape or volume.
Let's try a few questions about solids, liquids and gases!








