
If you spray deodorant or perfume, you will be able to smell it in the air. Someone standing on the other side of the room will take slightly longer to smell the scent that you've just sprayed. Why is this?
The answer is diffusion.
.jpg)
Let's learn what this means!
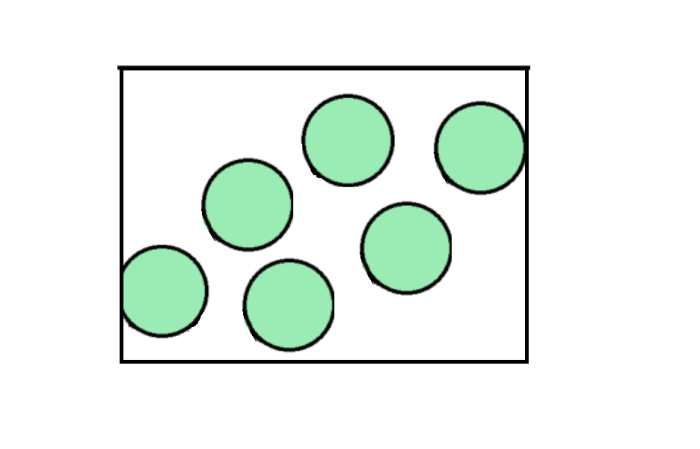
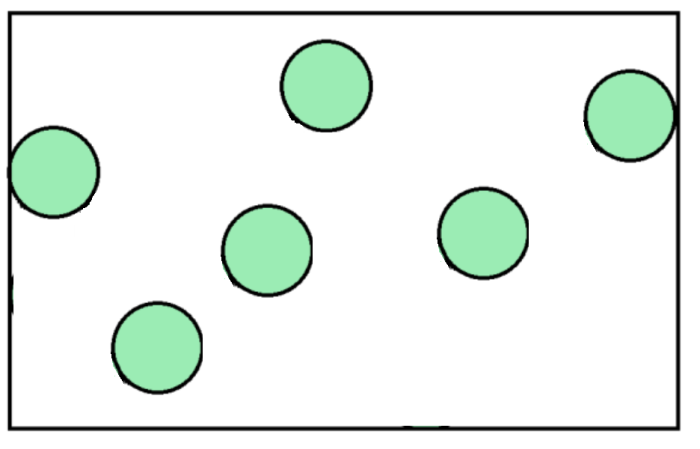
Diffusion is the spreading of particles from an area of high concentration to an area of low concentration.
The word 'concentration' means how many particles are in a given volume of space, so if they are very close together, it is a high concentration, and if they are far apart, it is a low concentration.
In the diagrams above, the picture on the left shows gas with a high concentration (as it is forced into a smaller space), while on the right, we can see that the particles have a lower concentration, as they have moved into a larger space and there is more room for them to spread out.
This is exactly why perfume spreads across a room. These substances are liquids, and when stored inside a bottle, the particles are very close together - therefore, they have a high concentration. When you spray the fragrance into the air, you are releasing the particles into a much larger space full of gas, where all the particles are much further apart (an area with low concentration). So, the particles of perfume will spread across it.
How can you speed up diffusion?
To make particles of fluid spread out more quickly, the most obvious thing to do is to give them more energy and make them move faster. To achieve this, you need to increase the temperature of the substance, so this is one factor that will affect the rate of diffusion. (If you've ever got ready for a PE lesson in a hot changing room, you may have noticed that the room smells more sweaty for this reason!)

Another factor is the difference in concentration. For example, if the area of high concentration was very similar to the area of low concentration, the rate of diffusion would be low. (The particles would not be in much of a rush to get there). On the other hand, if the area of high concentration was much greater than the area of low concentration, then the particles would race into it and spread much faster.
How does it know when to stop?
Diffusion does not continue forever. Once the particles in a fluid have spread evenly, the process stops. Diffusion will stop occurring when all the particles of a fluid reach a constant concentration. This is called being in equilibrium.
Phew - lots to get our heads around here!
Let's have a go at some questions, but remember that you can look back at this page at any point by clicking on the red help button on the screen.
.jpg)








