This activity will be testing your understanding of the three states of matter when applied to water.

For example: what is solid water, what has to be done to it to turn it to liquid water? What is 'water gas' called and can it become liquid water again? Does the outside temperature and pressure matter? These are some of the aspects of water we'll be exploring.
You probably already know that solid water is called ice. The freezing point of water is 0ºC. This means that ice will melt and water will freeze at this temperature. Water freezes because bonds start to form between its molecules, joining them together into a straight, rigid structure. This arrangement is called a lattice. (Melting ice consists of breaking these bonds to turn it back into water).
In most materials, the solid state is more dense than the liquid state, as a result of the particles becoming closer together. Water is unusual because ice has large spaces between its molecules, making it less dense than water. This is why it floats.

The boiling point of water is 100ºC. Water will evaporate at the boiling point. This time, it isn't bonds that break. There are forces between molecules (called intermolecular forces), and these are overcome at high temperatures, allowing the particles to escape from each other and move at high speeds in random directions. This turns the liquid into a gas.

We can refer to 'water gas' as steam or water vapour. 100ºC is also the temperature that steam will start to condense back into water if it is being cooled down from temperatures above the boiling point, as the molecules slow down, become closer together, and experience the forces that keep the material held together as a liquid.
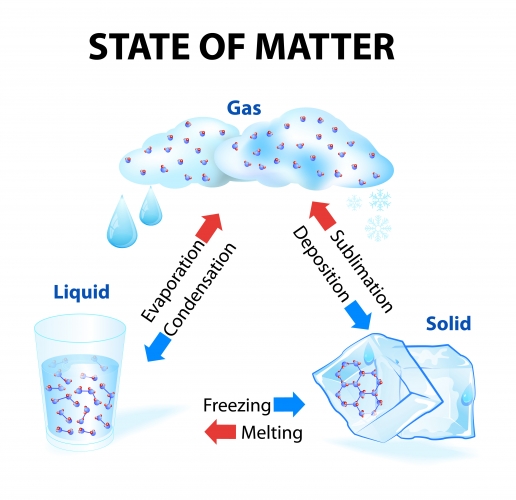
A summary of the three states of matter and the names of the changes are shown below:
Are you ready to try some questions now?