Solids, liquids and gases. These are probably words you have heard and used many times before. These are called the three states of matter.
Ice, steam and water - all made up of the same compound, but quite different in their properties. Could you classify them as either solid, liquid or gas?
.jpg)


That's right, ice is the solid state, water is the liquid state and steam is the gaseous state.
We can tell that ice is a solid because it has a fixed shape - it could be held in your hand (if you don't mind the cold!). Gases are more tricky to spot. This is because they are often colourless, and spread out all around us. But we can see the steam in the picture, and can tell it is a gas because it has no fixed shape and spreads up and out as far as it can. The water takes the shape of the glass it is in, but stays in the glass (unlike a gas would). It can be poured, and would take the shape of whatever container it was put in. Therefore, we know water is a liquid.

Although they are all made out of water particles, the solid, liquid and gaseous states of water have quite different properties. This is because of the way their particles are arranged.
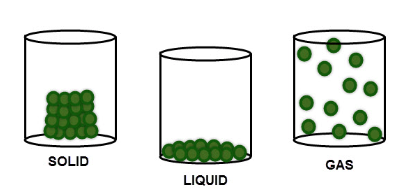
In a solid, the tiny particles that make up the solid are very close together in a neat regular arrangement, all touching each other and moving only by vibrating on the spot. They cannot move much at all, which explains why solids have a fixed shape.
The particles in a liquid are arranged in a more random way. They are close together, most of them touching each other, but there are some small gaps. So the particles can move around a little, over each other, allowing liquids to flow and be poured. This is the reason why liquids take the shape of the container they are in.
The particles in a gas are spaced very far apart, move around very fast and they are randomly arranged. Because of this, gases can be easily compressed, or squashed - they have no fixed shape or volume.
Think you know the difference between solids, liquids and gases now? Great! Then let's take a look at a few questions about them.








