Solids, liquids and gases are the three states of matter.
Solids have their own fixed shapes. They don't rely on a container to give them shape. They can be held, cut and sometimes reshaped.
Liquids and gases on the other hand, cannot be held in your hand - unless they are in a container, like a bottle or jar. Liquids and gases will take on the shape of whatever container or space they are in.

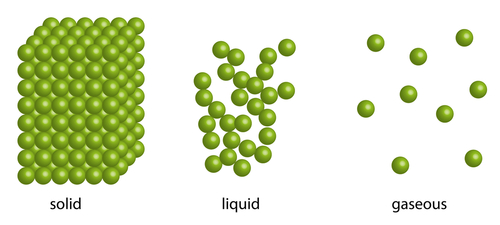
Liquids can take the shape of a container they are in because of the way their particles are arranged. The particles in a liquid are arranged in a random way. They are close together, most of them touching each other, but there are some small gaps. So the particles can move around a little, over each other, allowing liquids to flow and be poured. They will continue moving slightly over each other until they reach the sides of the container they are in.


Gases will fill the entire space of whatever container they are in - if the container is a room, they will spread out to each and every corner. Again, this is because of the particle arrangement in a gas. The particles in a gas are spaced very far apart, move around very fast and they are randomly arranged. It's almost as if they want to be as far away from each other as they can, so they will carry on moving and spreading out until the walls of a container stops them. If there is no lid on a container, the gas will escape! Also, because of this, gases can be easily compressed, or squashed.
Let's now take a look at a few questions about liquids and gases in particular.







