Separation techniques are used to split up mixtures into their different components. The types of separation technique used to accomplish this will depend on the states of matter in which we find the mixture's components.
| Separation technique | Useful for separating...... |
| Filtration | An insoluble solid from a liquid |
| Distillation | A solvent (liquid) from a solution |
| Crystallisation | A solute (soluble solid) from a solution |
| Fractional distillation | A liquid from a mixture of liquids |
| Chromatography | Coloured soluble substances |
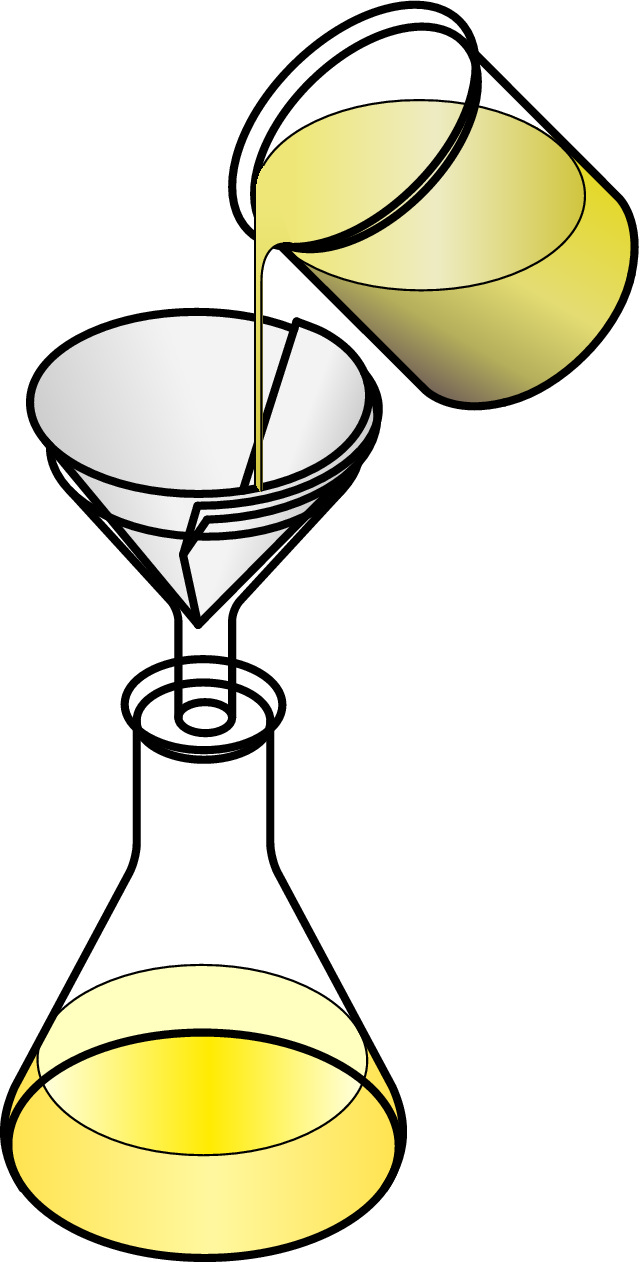
Filtration can be used to separate an insoluble solid from a liquid, for example if you had a mixture of sand and water. The mixture would be poured through filter paper in a funnel, through which the larger particles of insoluble solid would not be able to pass.
To separate a solution (a soluble solid or solute in a liquid or solvent) you could use either distillation or crystallisation.
Crystallisation is used to obtain solid crystals of the solute as the solvent evaporates slowly in an evaporating basin.
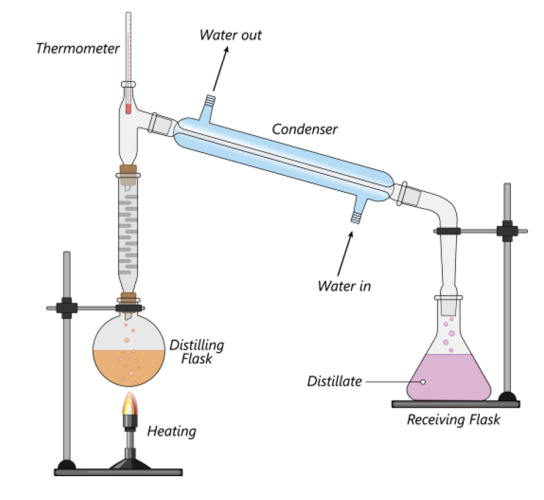
Distillation is used to separate a solvent from a solution, for example it can be used to obtain water from a salt solution. When the solution is heated, solvent vapour evaporates from the solution as it has a higher boiling point than the solvent, which is then cooled and condensed leaving behind the liquid solvent.
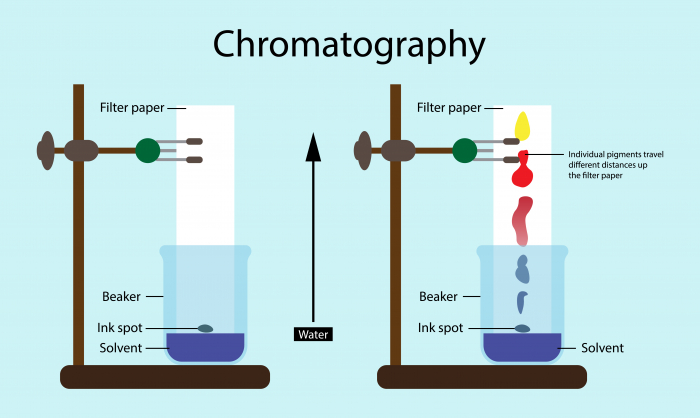
A solution made up of a mixture of soluble substances can be separated by chromatography, as the different dissolved substances have different solubilities so will move through the chromatogram at different rates.
For a mixture containing multiple different liquids, fractional distillation could be used. This works because the different liquids have different boiling points, so when the mixture is heated, the fractions vapourise and then condense when they reach their boiling point.
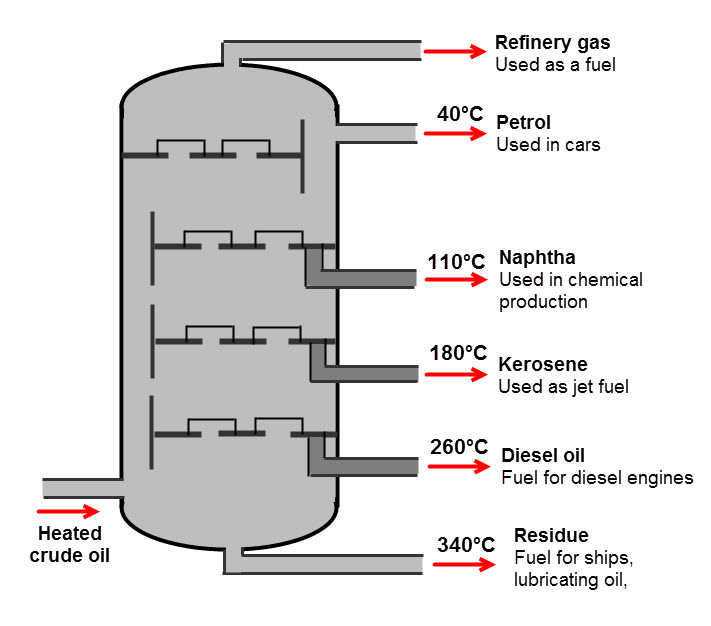
Do you think you would be able to choose the correct separation technique for various scenarios? Let's have a go!







