Heat is a form of energy that will always flow from hot to cold - for example, if a hot object is in contact with a cold object, the hot object's energy will be transferred to increase the cold object's temperature. If any object has a greater temperature than its surroundings, it will also transfer its energy to those surroundings, decreasing its own temperature as it does so.
When two objects reach the same temperature, or an object reaches the same temperature as its surroundings, the heat energy stops being transferred and this is called thermal equilibrium, as everything reaches a constant temperature.
You need to know the different ways that energy can be transferred between objects, which are laid out below.
Conduction

Protective clothing is needed by many people for various reasons. Every day, we wear clothing to protect us from the cold or the sun, but some people need safety clothing because they work under extreme conditions: for example, in high temperatures or in the cold sea.
The effectiveness of protective clothing depends on the material used and how its particles are affected by energy changes. Solid particles gain energy when heated, they vibrate more and pass the energy to each other as they bump into each other. This is called conduction, which means the energy flows through the solid materials. Hot solid particles need more space to vibrate, so solids expand, but when they cool down they contract.
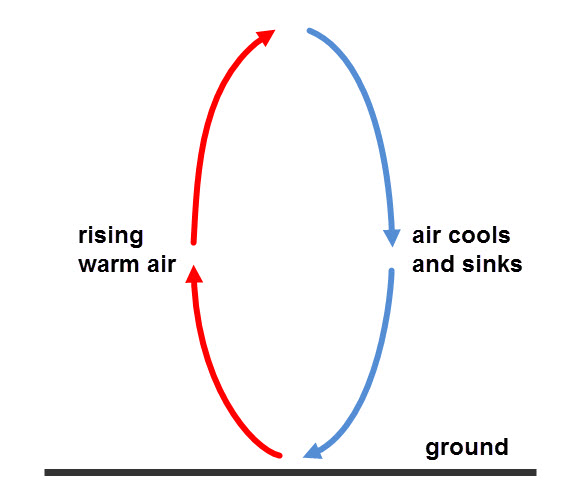
Convection
Liquid particles are further away from each other than in solids, so conduction does not take place very well and gases can barely conduct, as their particles are too far away from each other. Liquids and gases are fluids, so in fluids, heat energy travels better by convection. When a fluid is heated, the particles move around faster. The fluid also expands and becomes less dense, as the particles ‘spread around’, so there are fewer particles in the same space. The hotter part of the fluid starts to rise, and the cooler fluid around it takes its place.
Wind is an example of a convection current. Some parts of the Earth are warmer than others and they heat the air above them. This then expands and rises and the cooler air from cooler parts takes its space. In turn, the cooler air is also heated, expands and rises. The process then repeats itself, forming winds.
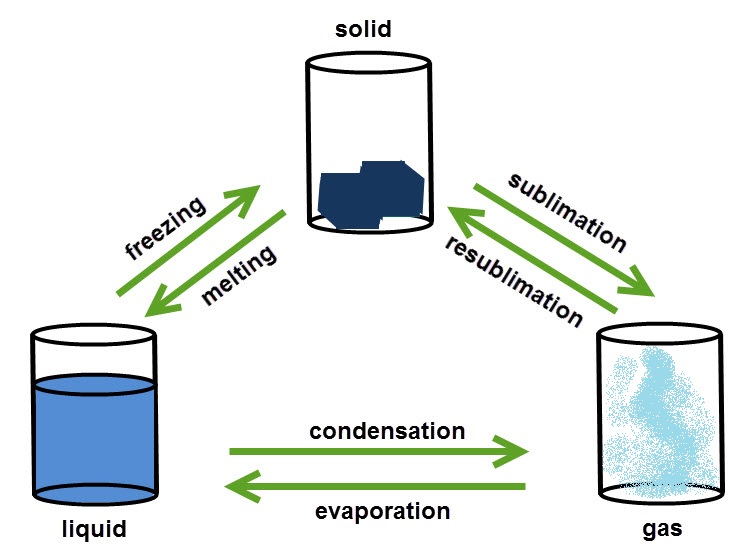
Changing state
This image shows the changes of state between solids, liquids and gases:
You can change substances from one state to another by heating or cooling. The freezing and melting point of a substance are always the same temperature. Changing from solid to liquid needs energy to break the bonds holding the particles together.
Radiation
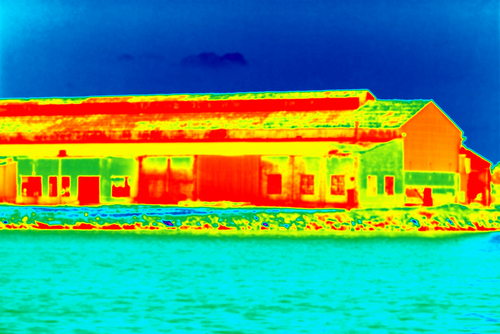
Heat energy can also be transferred as radiation, also referred to as infrared radiation. All hot things emit (give out) radiation. Dark, matte materials absorb (take in) more radiation than light and shiny ones, which reflect radiation more easily. Thermal imaging measures infrared radiation and converts it into temperature. Thermal imaging is used for filming at night, taking satellite images of the temperature of parts of the Earth, designing outdoor clothing and weather forecasting.
.jpg)
Radiation is different to conduction and convection because radiation does not need particles to flow. It is the only method of heat transfer that can travel through a vacuum (empty space). Radiation is the reason we can receive the heat energy from the sun, as it radiates its heat millions of kilometres through empty space.
Below is an example of a thermal image, showing the hotter parts of a building as red, orange and yellow and the cooler ones as blue and green.
Lots to remember here! Let's see how we can use this information in the questions that follow!