What are particles?
In physics, when we use the word 'particle', we may be referring to a couple of different things. Let's take a look:
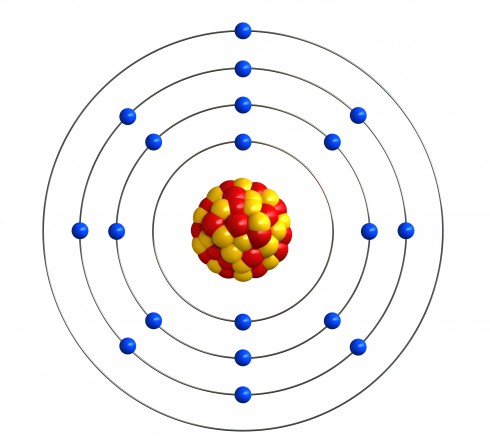
An atom is a single 'building block' of matter. In the diagram above, we have a single atom of a single element (in this case, it is potassium). The part in the middle is called the nucleus and the tiny dots circling it are electrons, but we don't need to go into that level of detail for this activity.
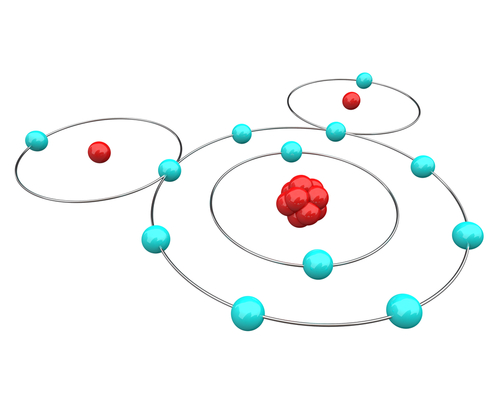
If you have more than one atom joined together, you now have a molecule. As you can see in the diagram above, molecules are larger and more complex than atoms. The diagram is showing a molecule of H2O, which is water. So, if you could see every little 'building block' of water under a powerful enough microscope, you would see two hydrogen atoms joined to an oxygen atom.
When we examine the way that all the matter inside a substance is behaving in particle theory, we do not consider whether the substance is made of atoms, molecules, or a combination of both. We simply refer to the little parts of the substance as particles. Our diagrams of particles do not need to include the nucleus, the electrons, or extra atoms connected to each other. They are much simpler illustrations that look like this:
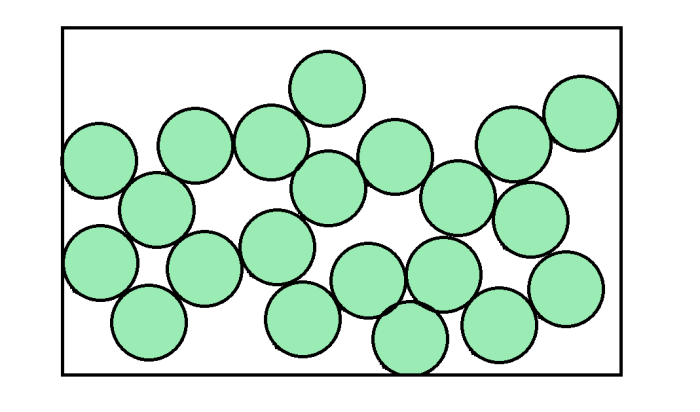
The circles may be representing either a set of atoms (i.e. a single element), or molecules (i.e. a compound such as water).
States of matter
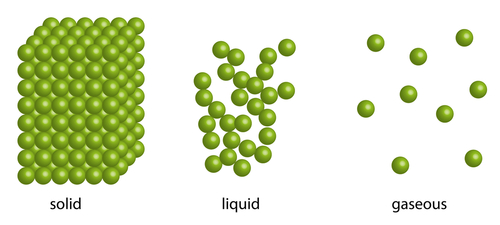
Solids, liquids, and gases are the three states of matter. They are all made of particles.
All of their properties are described using the particle model:
- The particles in solids are packed tightly together in straight rows and columns. This pattern is called a lattice. The particles vibrate about fixed positions, so they are always in motion. The forces of attraction between the particles are very strong, so they cannot flow.
- The particles in a liquid are still close together, but the forces of attraction are weaker than in solids. The particles in a liquid move randomly and do not follow a fixed pattern, which allows them to flow, and also change shape to fit any container.
- Finally, the particles in a gas move very fast and at random. Like gases, they can change shape and can flow. The forces of attraction between gas particles are very weak and they have very large spaces between each other.
Think you're ready to try some questions? Remember that you can always check back to this page by clicking on the red help button on the screen at any point.
.jpg)








