Acids and alkalis can be found in a chemistry laboratory, but also in our houses! Acids are substances with a tangy taste... that's right, like lemons. Citrus fruits like lemons and limes contain citric acid which give them their distinctive taste. They are also high in vitamin C which is also known as ascorbic acid. So, in fact, lemons contain two different sorts of acid! However, not all acids can be eaten.

At school, you're likely to use the following dilute acids: hydrochloric acid, sulfuric acid and nitric acid.
Alkalis can also be found in the laboratory or in our households. The most common laboratory alkali is sodium hydroxide.
Alkalis, generally, feel a bit like soap. Actually, they were used as cleaning products by ancient Arabs. They were made with ash and animal fat. Today, most cleaning products are alkalis, for example, toothpaste, soap and oven cleaners.

In a way, alkalis are the opposite of acids. There can be weak alkalis and strong alkalis. Strong acids and alkalis are very harmful and can corrode your skin, whereas weaker ones are irritants, which means they will give you a rash if they come into contact with your skin.
Here is the hazard symbol for strong corrosive acids.
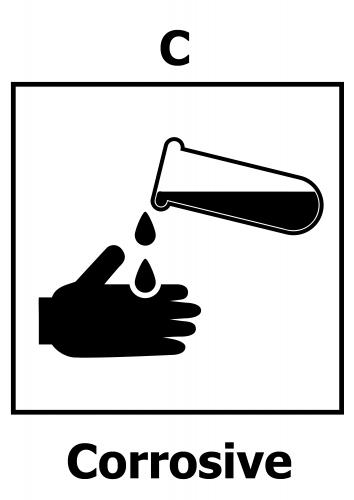
There is a way to identify whether a substance is an acid or an alkali - this is the pH test. You can see the pH scale below, which runs from pH0 (very acidic) to pH14 (very alkaline).
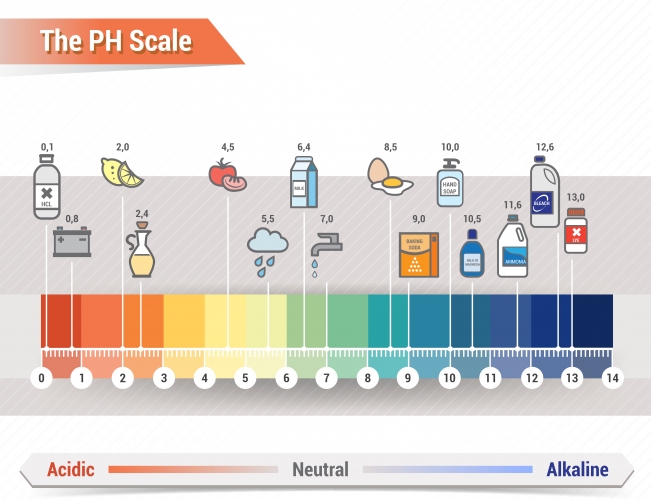
There is a special solution called Universal Indicator solution. When a few drops of Universal Indicator are added to a substance, it turns a different colour. This colour matches one of the colours on the Universal Indicator scale above and that's how you determine how acidic or alkaline a substance is and so what its pH is.
Strong acids are red on the scale (pH 1-3), whereas weaker acids are yellow (pH 4-6). Strong alkalis are dark blue or even purple on the scale (pH 12-14) and weaker alkalis are lighter blue (pH 8-11). The area in between, where it is green, is pH 7 - this is what colour neutral substances would go. Neutral substances are neither acidic not alkali and the most obvious neutral substance is water.
Are you ready to try some questions on this now?








