Fractional distillation can be used to separate a mixture of liquids with different boiling points.
Air is a mixture of nitrogen, oxygen, carbon dioxide and some other gases. The composition of air is shown in the pie chart below.
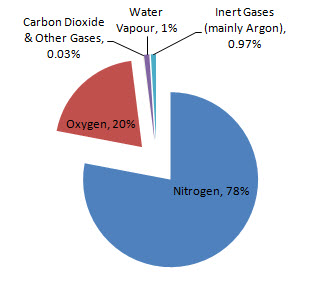
To separate this mixture of gases, the air must first be cooled to -200oC, which turns it into a liquid. It is then warmed up slowly and each of the liquids boil off at a different temperature so they can be collected and removed.
.jpg)
Fractional distillation can also be used to separate crude oil into the many compounds that it is made up of. Crude oil is a mixture. This is very important as crude oil contains some very useful compounds such as petrol and diesel.
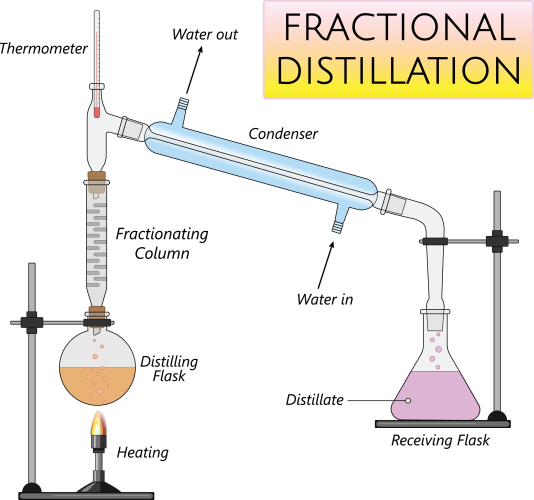
In the science laboratory, a fractionating column along with a condenser is used to separate the different groups or fractions of oil. You can see how this apparatus is set up in the lab in the image below:
The products of fractional distillation of crude oil are shown in the diagram below:
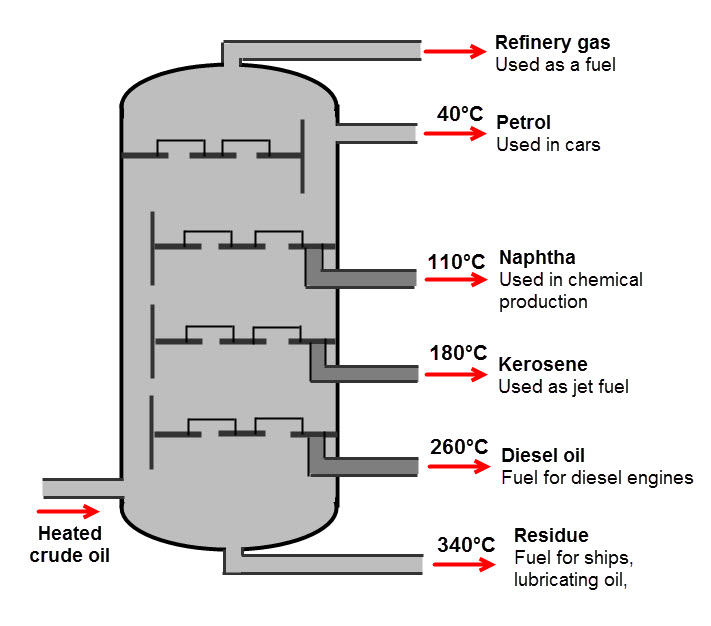
We can see that from crude oil there are lots of different, useful fractions produced. This is a reason why fractional distillation is so great!
Shall we try the questions on separating mixtures of liquids?








