One word you will have heard many times before is the word 'molecule'. But if someone asked you to describe what a molecule was, could you?

Tricky isn't it?
Before we can start thinking about what a molecule actually is, we have to start with something even smaller. Atoms. Everything you can (and can't see!) is made of atoms. Atoms are the building blocks of everything. They are super-small, and can't be seen with the naked eye. A bacterium is about 10,000 times bigger than an atom, and we need a microscope to even see them! So atoms are really, really small!
Atoms can exist on their own or be joined to other atoms of the same type to form a molecule. They can also form bonds with other atoms of different types to form a molecule called a compound.
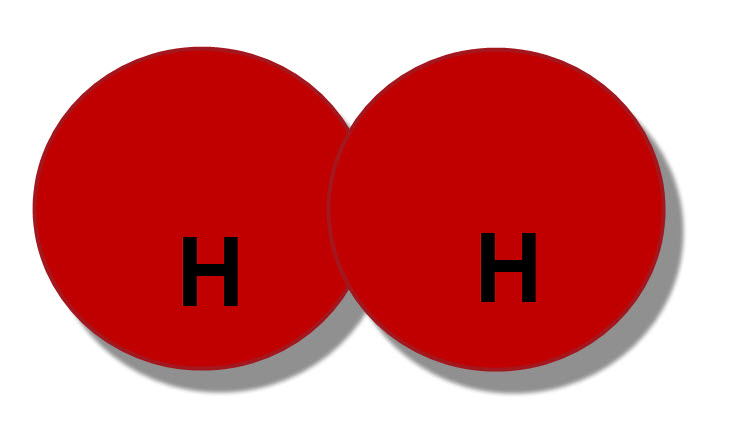
The atoms of elements such as hydrogen, nitrogen and oxygen join in pairs to make molecules. A molecule of hydrogen would contain just hydrogen atoms, so in a diagram, they'd both be the same colour.
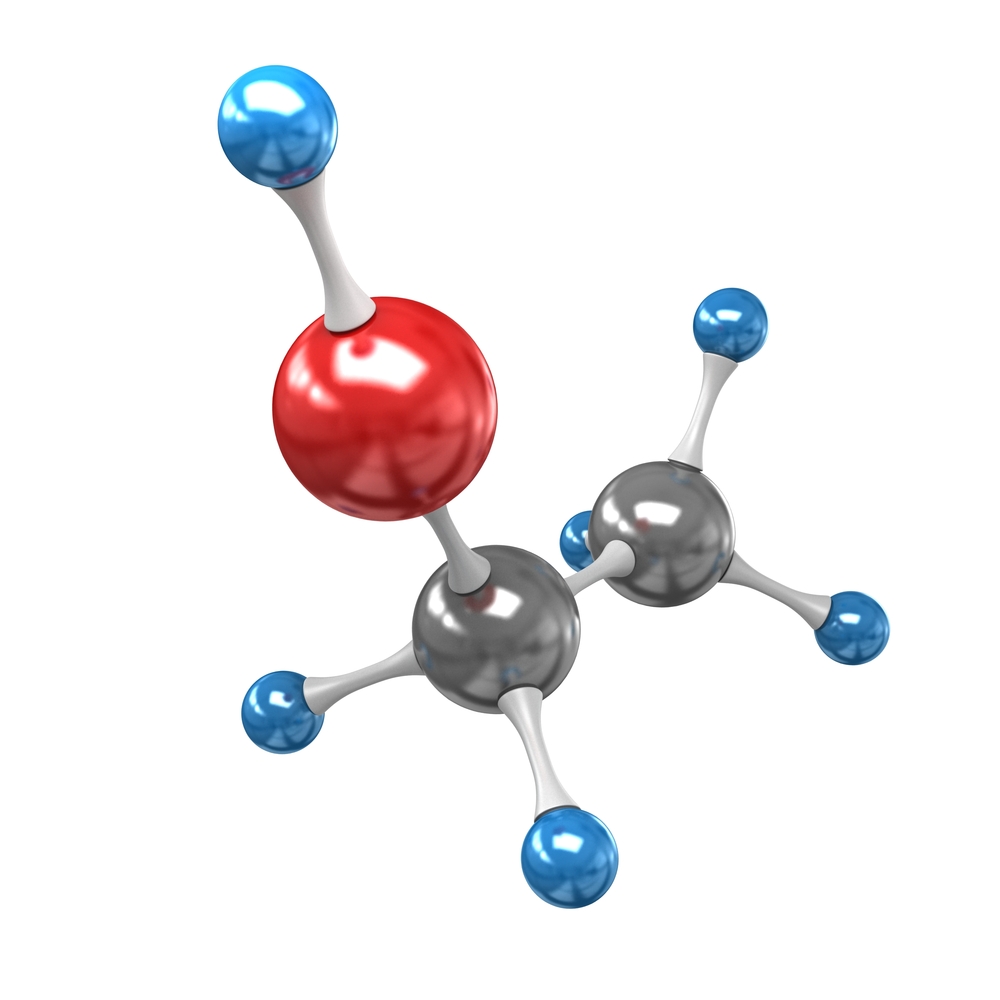
The model below shows the atoms in a molecule of ethanol, which is a compound. The circles represent individual atoms. Different coloured circles represent different types of atoms. So we can see that ethanol is made up of three different types of atom.
So, let's go back to our initial question. Can you describe what a molecule is? Yes! A molecule is made of two or more atoms chemically bonded together.
Ready for some questions on molecules? Great stuff, let's take a look together........








