Atoms are the building blocks of everything. You can think of them as bricks if you like. They can exist on their own or be combined in different ways to create houses, schools, walls, patios, towers and more. And there are lots of different types. However, unlike bricks, atoms are super-small, and can't be seen with the naked eye.

.jpg)

Atoms have a radius of about 0.1 nanometre! If you think that's small, how about if we told you that there are actually even smaller particles, called subatomic particles, inside an atom. Wow! We can use models to show what the arrangement of these subatomic particles would look like.....
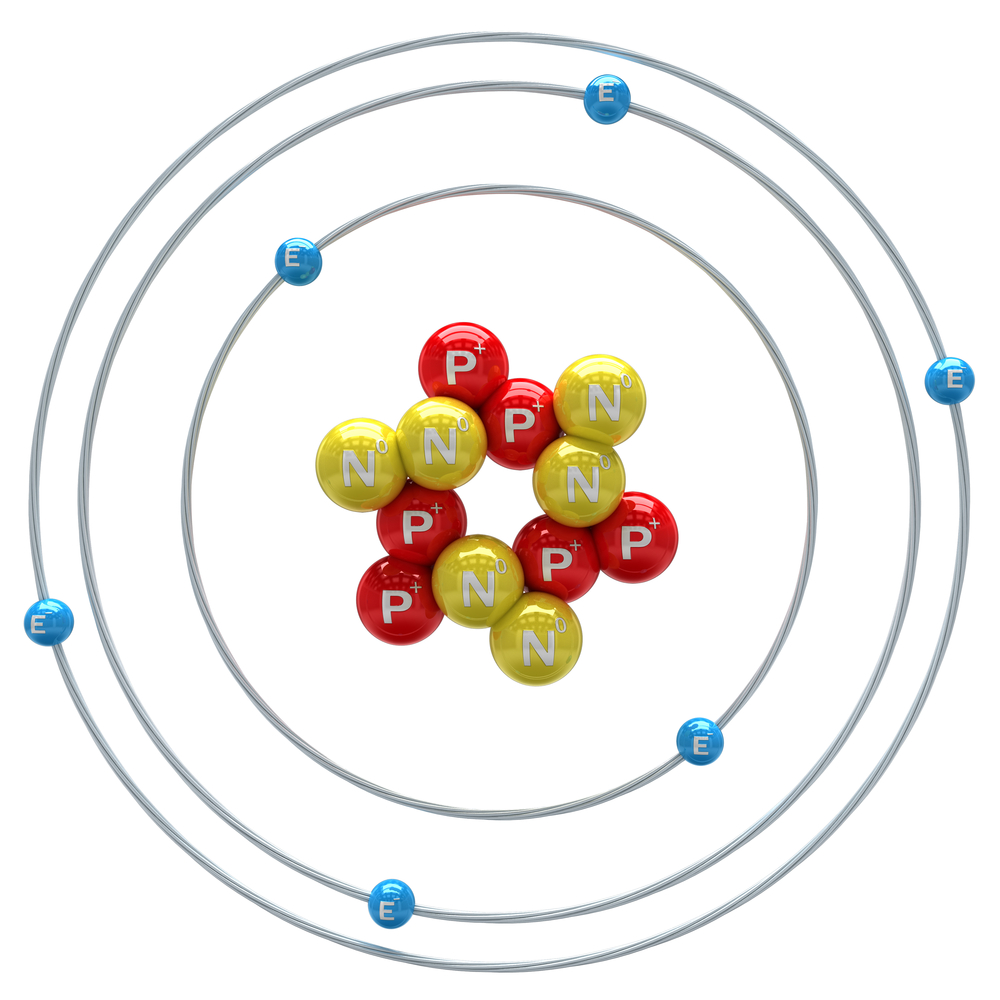
The particles labelled P are protons, the particles labelled N are neutrons and the ones labelled E are electrons.
But let's zoom back out slightly as that's more complicated than we need to think of right now. We can show atoms more basically as small coloured circles - and it's always circles, never squares or triangles. Atoms of the same element are the same colour and size. Different types of atoms would be shown as different coloured circles.
Atoms can exist on their own or joined to other atoms of the same type to make up an element, or can form bonds with other atoms to form molecules.
For example, carbon atoms can be joined to other carbon atoms to form super-strong diamond, or joined to oxygen to make carbon dioxide gas, or even joined with oxygen and hydrogen to make a liquid, alcohol. No matter what, the atoms of carbon remain the same. They cannot be destroyed or changed themselves, they remain the same size - it's just their arrangement that can change.
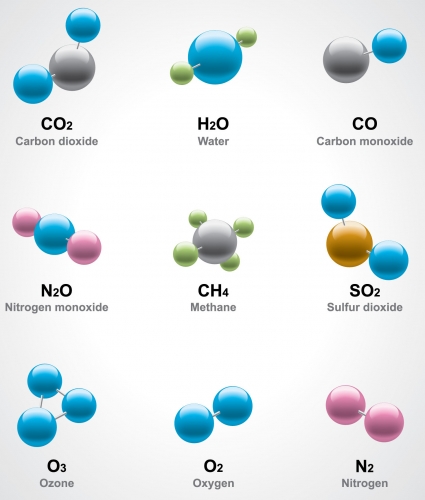
The image above shows various molecules. The circles represent individual atoms.
We can see that some molecules are made from only one type of atom, such as oxygen and ozone. This is how some atoms like to go around. Others are just happy to exist as single atoms. But some of the molecules above are made of two or more different types of atom, such as methane or nitrogen dioxide - the ones which are made of two or more different types of atoms are called compounds.
Ready for some questions on these little things we call atoms? Great, let's go........