Mike: My teacher's asked me to work out the relative formula mass of water. How do I do that?
Jenny: Easy! You just need to find out the mass of each atom in the substance, in this case water (H2O), and add them all up.
Mike: So do you need a teeny weeny set of scales to weigh each little atom?
Jenny: No silly - the masses of each atom of an element are written on the periodic table. So you can put those tiny weighing scales away!
.png)
Take a look at the section of the periodic table above. Each element has a symbol and two numbers next to it. Remember what these numbers tell us?
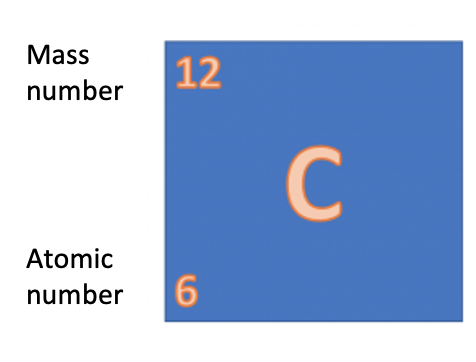
So let's go back to the question Mike's teacher asked him - what is the relative formula mass of water (H2O)? Well, we can see on the periodic table that hydrogen has a mass of 1 and oxygen has a mass of 16.
So in H2O there are two atoms of hydrogen and one atom of oxygen, so the relative formula mass of water would be:
1 + 1 + 16 = 18
Note: There are no units for relative formula mass.
The Law of Conservation of Mass states that during a chemical reaction the total mass of the reactants is equal to the total mass of the products. Mass is always conserved.
In which case, the sum of the relative formula masses of the reactants will be the same as the sum of the relative formula masses of the products. Make sense?

Let's see below:
iron + sulfur → iron sulfide
Fe + S → FeS
Mass numbers: Fe = 56, S = 32
There is one atom of each element on each side of the equation:
56 + 32 → 88
Law of Conservation of Mass states the RFM of reactants (88) is the same as the RFM of products (88)
Yesssss! Mass is conserved!
Ready to give this a try?