A mixture in chemistry is like a cake mix in the kitchen - all the different components are in the same bowl, but you can still pick out the chocolate chips if you really want.

In chemistry, we say that a mixture is:
Two or more substances, mixed but not chemically joined
To put this a simpler way, it is two or more substances that are present in the same container but are not joined to each other (by chemical bonds).
Shown below is a mixture of elements. An element contains only one type of atom and, because these atoms are not joined to each other (combined), it is a mixture of elements.
.jpg)
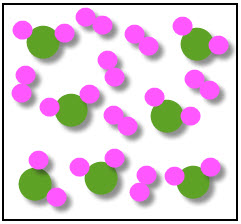
Shown below is a mixture of compounds. A compound is made of two or more elements chemically joined together.
Look closely at the diagram and you will see the two different compounds but, because they are not joined to each other, it is a mixture of compounds.
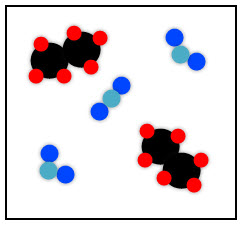
Shown below is a mixture of an element and a compound. The two pink atoms combined represent the element. The atoms are the same and so this is an element. The compound is made of different atoms bonded together.
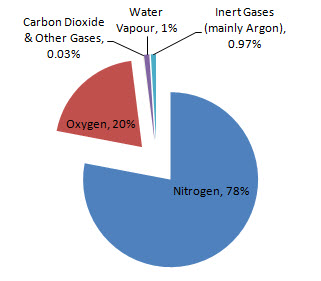
Air is a mixture of elements mainly nitrogen and oxygen, but also contains a small number of other gases such as carbon dioxide, and the noble gases.
Want a bit more help with remembering what elements and compounds are, before you begin? Why not watch this short video?








