The periodic table contains all of the known elements, and sorts them into groups of elements with similar properties. Each element has a unique symbol. But have you noticed that there are two numbers next to the symbols too?
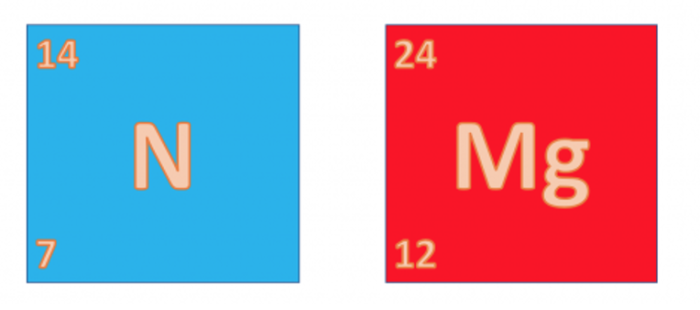
The smaller of the two numbers is called the element's atomic number. It tells you how many protons are in the nucleus of an atom of that element. No two elements will have the same atomic number. It is unique to that element. So as you can see above, the atomic number of nitrogen is 7 and the atomic number of magnesium is 12.
The second number, the larger of the two, is called the element's mass number. Since the mass of an atom comes from the protons and neutrons in an atom, the mass number is the total number of protons and neutrons in the nucleus of an atom of that element. So as you can see above, the mass number of nitrogen is 14 and the mass number of magnesium is 24.
If you know the mass number (number of protons + number of neutrons) and the atomic number (number of protons), then you can calculate the number of neutrons:
number of neutrons = mass number – atomic number
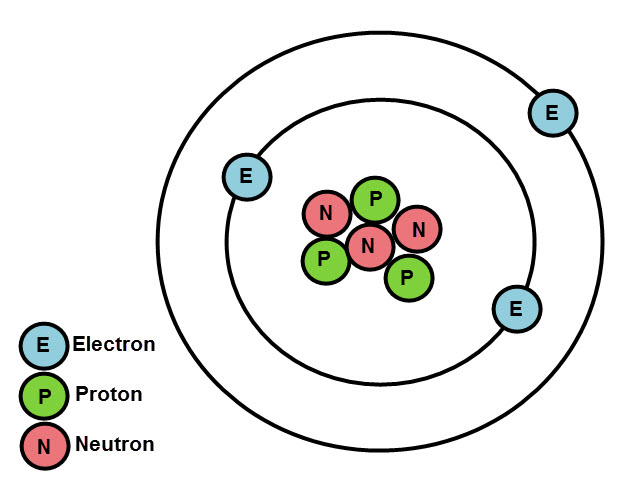
Remember, the number of protons and number of electrons is always equal in an atom, so the atomic number and mass number can tell us exactly how many of each subatomic particle there is in any atom of an element. Clever hey!
Let's try some questions.







