Ever wondered why your urine is often darker and more concentrated in the summer? Probably not, it's not the nicest thing to be thinking about! But often it's because we tend to sweat more in the summer months, so we lose a lot more water. Our kidneys then will try and keep a hold of more water for many of our important processes, resulting in more concentrated urine! This movement of water into and out of our cells is called osmosis.
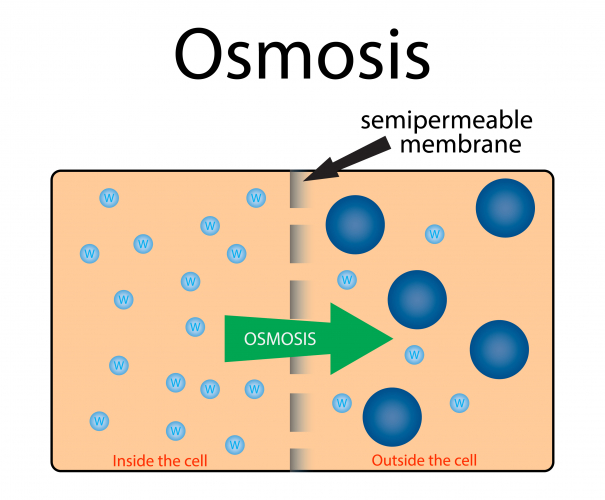
Osmosis is the diffusion of water molecules from a high concentration of water to a low concentration of water, across a partially permeable membrane.
A partially permeable membrane has holes or pores in it that allow water molecules through but are too small to allow larger molecules through.
During osmosis, water molecules diffuse from pure water or dilute solution to more concentrated solutions.
Dilute solutions have a high concentration of water molecules so have a high water potential.
Concentrated solutions have a low concentration of water molecules so have a low water potential.
Osmosis in organisms
Plant and animal cells are surrounded by a partially permeable plasma membrane. This allows water and other small molecules to diffuse across. Plant cells additionally have a strong cell wall surrounding the membrane, which offers support and protection. Animal cells don't have a cell wall. This means they respond differently to plant cells in the gaining and losing of water.
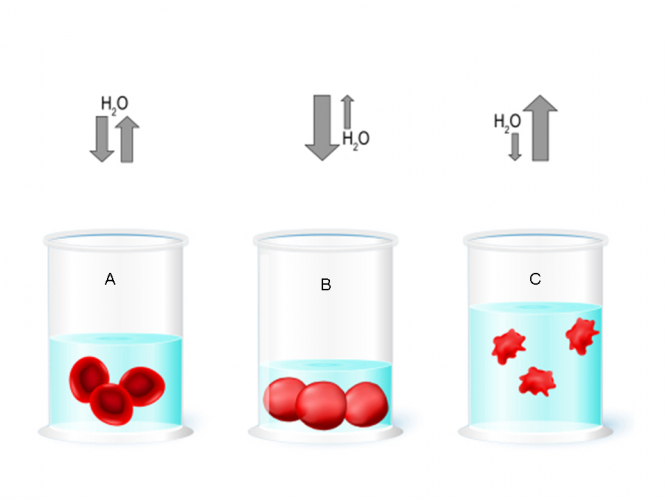
In dilute solutions, osmosis can cause animals cells, such as red blood cells, to swell up and sometimes even burst (beaker B in the image above). This is because water will move from a high water potential outside the cell to a low water potential inside the cell. In concentrated solutions (where there is less water present), water loss causes the cells to shrink (beaker C). Animal cells need to maintain a balance, this means that the water concentration both inside and outside the cell are equal (beaker A).
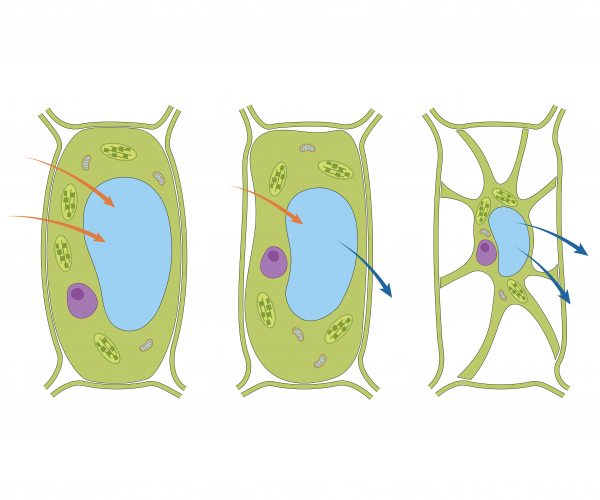
Plants require water in order to photosynthesise. The roots of a plant contain root hair cells which are specialised cells that increase the surface area of the cells for maximum absorption of water by osmosis. In pure water, plant cells will take in water via osmosis and become firm or turgid. In a concentrated solution (not much water present), the cell loses water and starts to shrink and becomes flaccid.
In humans, the concentration of water and salt in the blood is controlled by the kidneys. The kidneys ensure that our bodies have the right concentration of water and get rid of the excess water as urine.
Calculations Involving Osmosis

Osmosis can be demonstrated using cubes of potatoes of roughly the same mass. By placing the cubes in different concentrations of sugar solutions, the cubes might gain or lose mass, or might even stay the same mass.
Scientists will be able to calculate the percentage change in mass to see how much mass was gained or lost - converting to percentages often makes for an easier comparison. Percentage mass is calculated by using the following equation:
(Final mass - initial mass) ÷ initial mass x 100
For example, a potato cube has an initial mass of 1 g. After placing it in a sugar solution for 30 minutes, its mass was 1.1 g. Its percentage change in mass is 10%.
(1.1 g - 1 g) ÷ 1 g x 100 = +10%
The plus sign shows there was an increase in mass. A minus sign shows a loss in mass.
The rate of water uptake can also be calculated. This gives an indication of how much water is absorbed in an hour. The rate of uptake is calculated using the following equation:
Change in mass (g) x (60 minutes ÷ duration of time in minutes)
Using the above example of the potato cube:
0.1 g x (60 mins ÷ 30 mins) = 0.2 g/hour
In the following activity, you will interpret data relating to osmosis.







