Without enzymes, we could not function at all!
Every chemical reaction our body needs to survive, such as respiration, digestion and even thinking, all depend on fast reactions maintained by enzymes.
Both chemistry and biology are mad about enzymes, so let’s get going!
What are they?
Enzymes are a type of protein made by cells.
Reminder: A protein (or polypeptide) is a polymer of a chain of specifically ordered amino acids which is then folded.
The amino acid chain is folded and held in place by chemical bonds, giving a unique 3D shape.
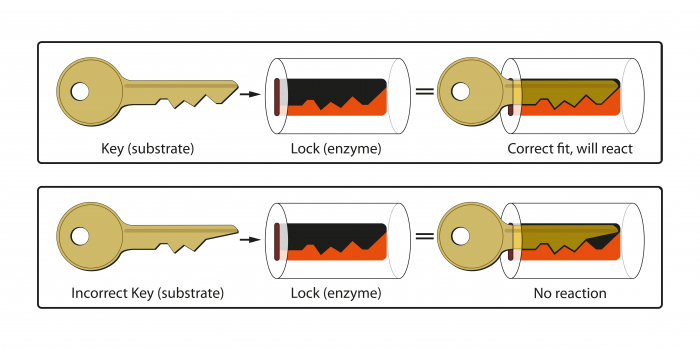
For enzymes, the final structure always includes an area called an active site, and the shape always matches only one molecule!
So NO FIT = NO REACTION!
What do they do?
They are biological catalysts!
A catalyst speeds up reactions without being used up.
How do they work?
They work using the lock and key hypothesis:
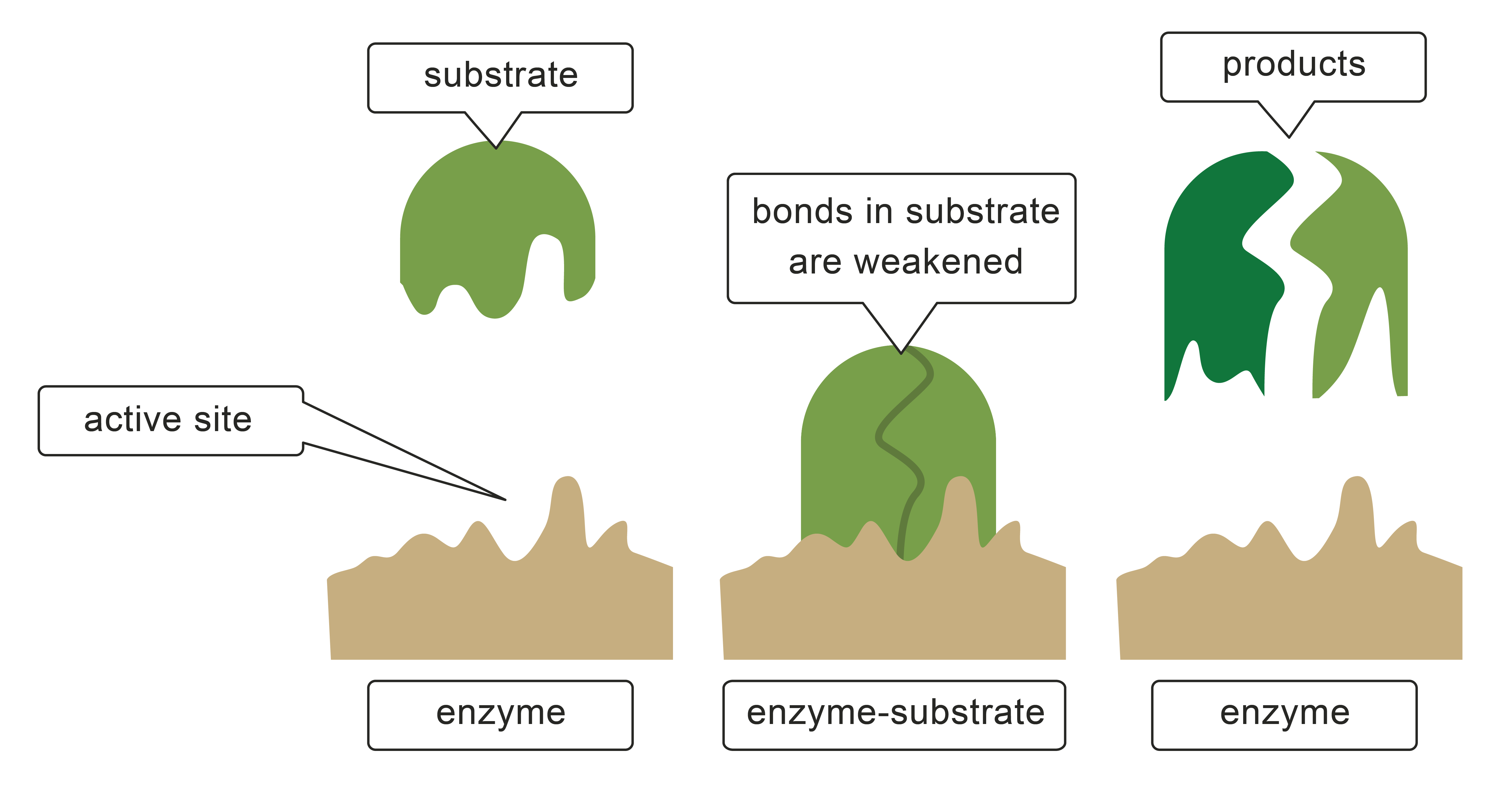
The correct specific enzyme needs to be present, with its active site being the right fit for the starting ingredient or substrate.
The substrate attaches to the active site, and this match is described as the enzyme-substrate complex.
The speed of the reaction increases, a product is made and the enzyme is reused.
Reaction rate factors:
Every enzyme has its own ideal temperature and pH!
Temperature
There are three key points:
Every enzyme has its optimum temperature.
Changing the temperature will change the number of successful collisions.
Too much heat can denature the active site.
So let's break it down:
The chemical bonds holding the amino acids in their 3D shape are sensitive to levels outside the enzyme’s ideal range, and most boiling temperatures damage an active site beyond repair.
So this is where we see the process of denaturation!
Being denatured means that the active site starts to break down because too high a temperature is breaking the chemical bonds.
Denaturation can lead to the whole reaction slowing down and no product being made.

Also, enzyme-substrate complexes are dependent on collisions between the enzyme and the substrate.
The enzyme and the substrate need energy to move around, so they have a better chance of hitting each other and producing a reaction.
But if the temperature dips too low, there is less movement and so less chance of successful collisions being made, slowing down the reaction.
And let's not forget pH!
This can also have a big impact on the active site shape, again because of the sensitivity of the chemical bonds between the amino acids in the folded 3D structure.
If the pH makes a huge change outside the enzyme’s comfort zone, the active site will be denatured, the substrate won’t be able to fit, which, you guessed it, slows the reaction right down!
Let's move on to some questions now.








