In 2011, an earthquake hit Japan registering as a 9 on the Richter scale – the largest earthquake in Japan's history and the fourth biggest in the world. This created a tsunami six metres high and killed over 15,000 people. One of the places hit by the earthquake was a town called Fukushima where they had a nuclear reactor. Unfortunately, the damage caused by the tsunami stopped the reactors from working correctly and three of them suffered meltdowns, spewing radioactive material into the environment. People were evacuated from their homes and a five km exclusion zone was set up to protect people from the danger of the radiation. But what is radiation and how is it caused? This is what we will be looking at in this activity.
There is a level of background radiation around us all the time. There are many sources of background radiation and this pie chart shows some of them:
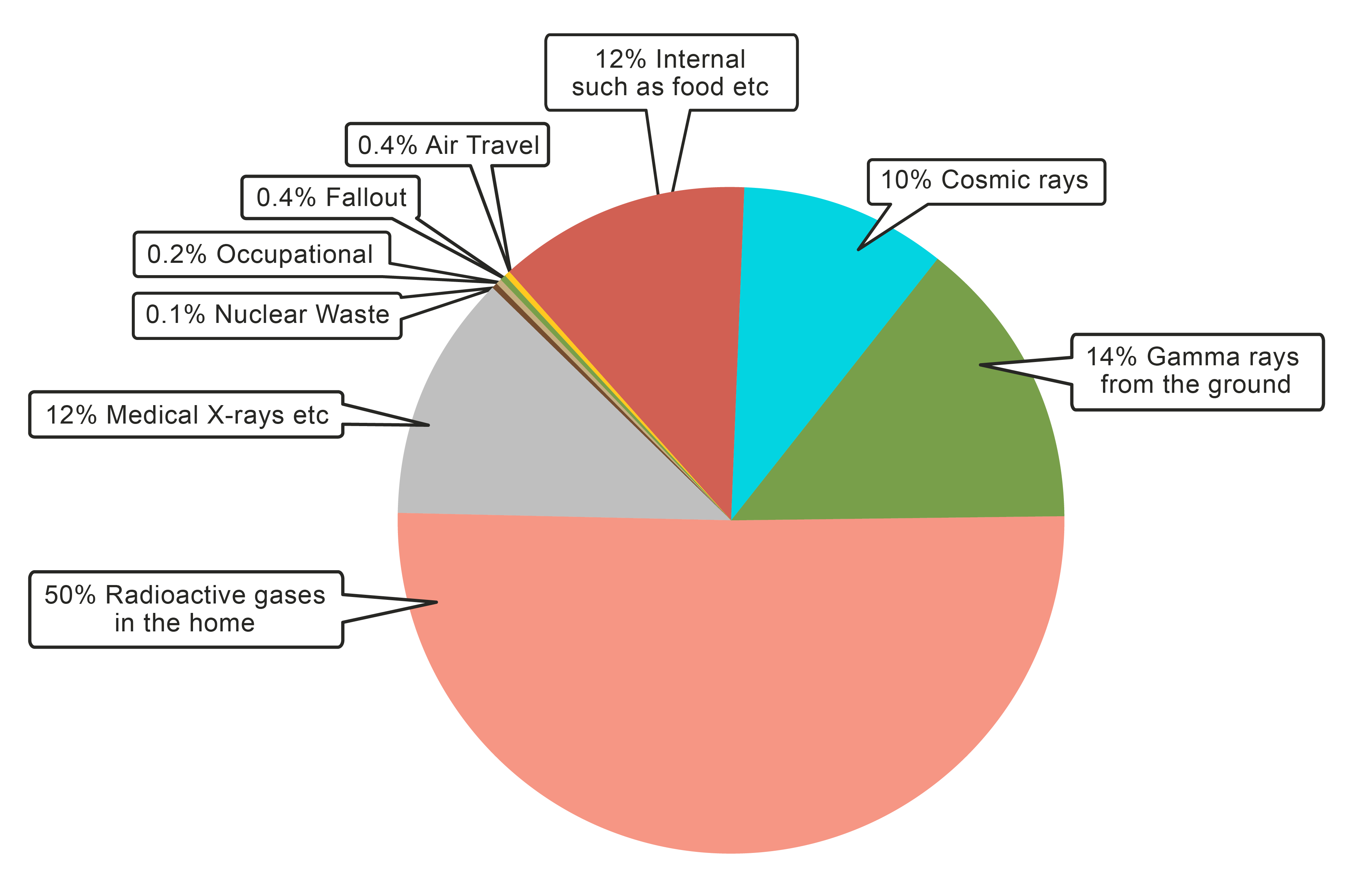
When the nuclear commission was helping with the clean-up from Fukushima, they took a range of readings looking for different types of radiation. They needed to do this because different types of radiation cause more or less damage depending on where they are. Let's look at the different types of radiation in detail:
Alpha (α):
Alpha radiation causes the most ionisation, but only travels a very short distance before it stops (about 15 cm). It can be stopped by the skin and so it is safe if it remains outside your body – but if you inhale it or drink it, then it can be deadly. A Russian spy, Litvinenko, was killed by a VERY small amount of alpha radiation being put in his tea.
Alpha radiation is made of 2 protons and 2 neutrons – it is a helium nucleus. This gives it a charge of + 2, meaning that it will rip electrons off things it comes near to, causing damage to them – but it can only do this twice.

Beta (β):
This is moderately ionising and can travel up to about one metre. It is not really safe inside or outside your body, so it’s best to stay away from it, full stop – but it is less likely to kill you than alpha, unless you have a high dosage of it.
It is made of a fast-moving electron usually spat out from the inside of a nucleus. It causes damage by hitting other electrons and removing them from their atoms.

Gamma (γ):
This is a strange one because it is a wave, not really a particle (although it is carried by photons). It is the least ionising of all of the three that we have looked at so far because it needs to hit stuff at just the right speed to cause damage. So, as a result, it normally just passes through everything - safe on the inside and the outside, unless there is a lot of it.
Neutrons (N):
These are the ones that you need to be afraid of and the ones that they were most concerned were being released from Fukushima. They are made of a neutron (who would have guessed, right?) and they are so dangerous because if they collide with a nucleus, then they will make the nucleus itself radioactive, causing it to release alpha, beta and gamma radiation.

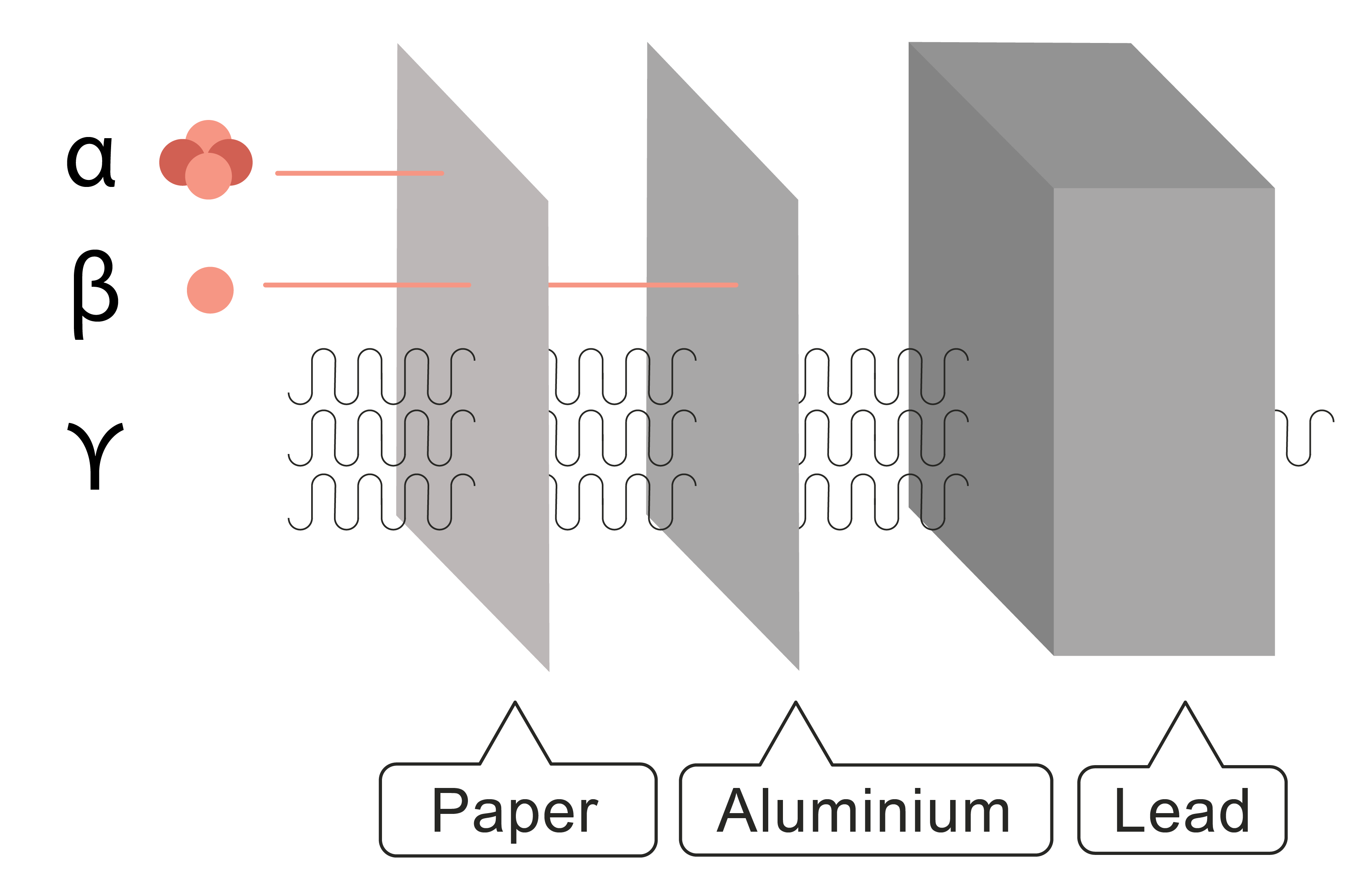
Alpha, beta, gamma and neutron radiation come from the nucleus of a radioactive atom. Alpha can be stopped by a thin piece of paper, beta penetrates the paper but can be stopped by a 3 mm thick piece of aluminium and gamma can penetrate a 3 m thick, lead block or concrete. The diagram shows this in more detail:
So, now we need to look at how to write nuclear equations. Don’t worry – these are the easiest equations you will ever do!
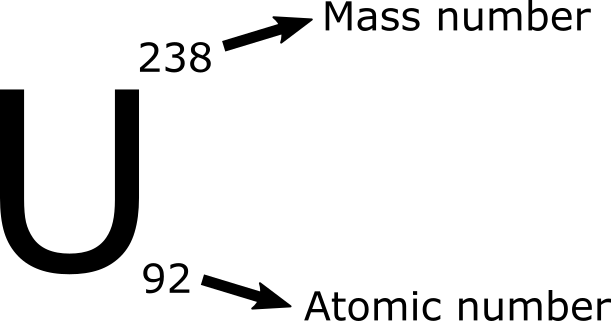
The first thing you need to know is what the atomic number and mass number are. Mass number is the number of neutrons and protons in the nucleus and atomic number is the number of protons only.
When we have an alpha decay, the original atom needs to lose 2 protons and 2 neutrons – this means that it will take 4 from the mass number and 2 from the atomic number. This, in turn, makes a new atom – thorium in this case – with a mass number of 4 less and an atomic number of 2 less.
Key point – the number needs to be the same on each side!

When doing beta decay, a neutron turns into a proton. This will not change the mass number, but it will change the atomic number. A beta particle is an electron, so has a mass number of 0. It also has an atomic number of -1 because it has the opposite charge of a proton. This nuclear equation looks like this:
Key point – the number needs to be the same on each side!

And that's it - now questions!








