Supposing we had a sample of a chemical, but we didn't know what the chemical was. How could we find out?
One way would be to take a small sample of the chemical and burn it. Many chemicals burn with distinctively coloured flames, which can give us clues to the chemicals involved. For example, the green part of this flame tells us that there are copper ions (Cu2+) in the chemical.

The same idea works for other metal ions, like this:
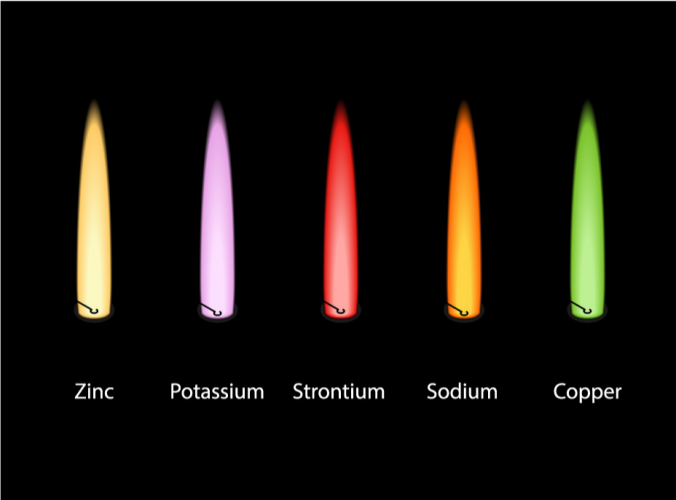
The ions are giving off light - this is also called emission. By understanding why different atoms emit different colours of light, we can see how to use this idea to identify different atoms.
What is emission spectroscopy?
This diagram outlines the method:
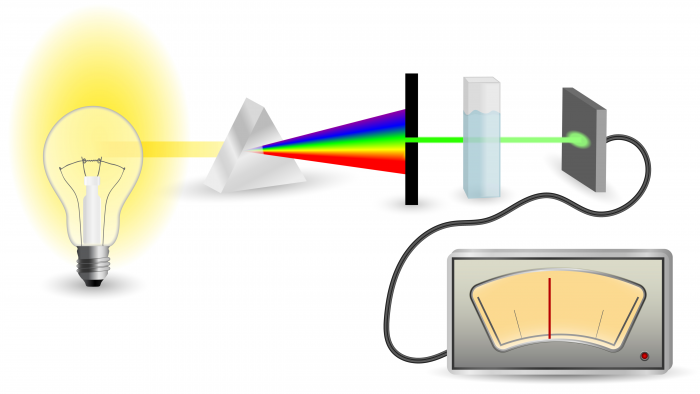
In this case, we are doing spectroscopy on the wire inside the light bulb. By passing electricity through it, energy is transferred to the atoms in the wire, and they emit light. Another way of transferring energy to the atoms is by heating them in a flame. By passing the emitted light through a prism, or a diffraction grating, the beam of light is split into separate colours. The wavelength and intensity of the colours is then recorded, giving us the emission spectrum.
Different elements have different emission spectra. For example, this is the spectrum for hydrogen:
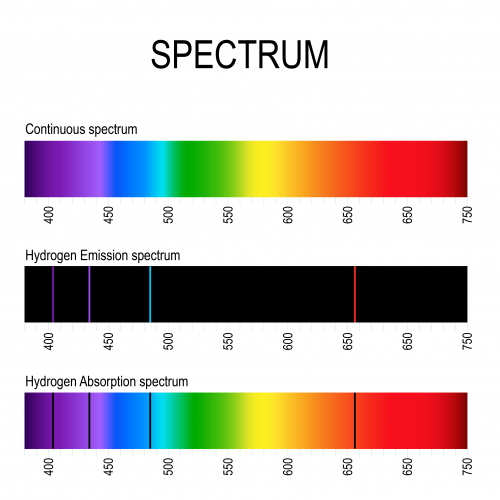
If we have a mystery element, we can record its spectrum, then compare it with the known spectra of different elements until we find one which matches.
Why do atoms emit (and absorb) light?
Look at these two pictures of sodium atoms. How are the white and yellow atoms different?
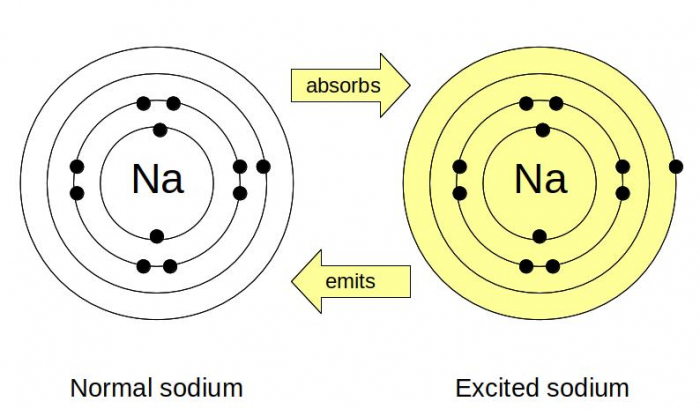
They're both sodium atoms and both have eleven electrons. The normal sodium atom (the white one, on the left) has the electrons in the usual configuration - 2 in the first shell, 8 in the second and 1 in the third. This is also called the ground state of sodium. In the excited sodium atom (the yellow one, on the right), the electron in the third shell has moved into the fourth shell. For this to happen, the atom must have absorbed some energy. When the electron jumps back to its normal (ground state) position, the energy is released again. If the atom absorbs energy, we say it is excited; if it releases energy, we say it is de-excited.
The energy transfers in and out of the atom as a particle of light, called a photon. The exact colour of the photon depends on the amount of energy being absorbed or emitted, which depends on the exact energy levels of the different electron shells. What that means is that every different atom or ion has a unique set of colours of light which it can absorb and emit. The colour we see in a flame test is a combination of all those colours.
Emission spectroscopy is a way of making flame tests more precise. Instead of just looking at the colour, we break it into its components. This means that we can be much more certain about exactly what elements are present in a sample. It's also much easier to detect elements at very low concentrations. Their colours might get swamped by brighter colours in a flame test, but will show up separately in an emission spectrum.
Let's move on to some questions now.








