
EdPlace's GCSE home learning biology lesson: Osmosis
Looking for short lessons to keep your child engaged and learning? Our experienced team of teachers have created English, maths and science lessons for the home, so your child can learn no matter where they are. And, as all activities are self-marked, you really can encourage your child to be an independent learner.
Get them started on the lesson below and then jump into our teacher-created activities to practice what they've learnt. We've recommended five to ensure they feel secure in their knowledge - 5-a-day helps keeps the learning loss at bay (or so we think!).
Are they keen to start practising straight away? Head to the bottom of the page to find the activities.
Now...onto the lesson!
Maximising Your Marks
Biology is often seen as the easier of the sciences by many students, but this is a mistake and teachers often find that students will invest in learning the topics they understand but avoid the more difficult aspects of the subject. Osmosis is the most common area where students miss marks in Biology exams and this can in many cases lower the final grade outcome of students.
While many find Biology the most approachable of science specialisms there are certain areas which are particularly tricky to understand. Osmosis is an area which is greatly developed upon in A-Level Biology due to its high level of demand. This makes it vital to understand for any student considering A-Level Biology in Key Stage 5, and, a key topic for all to learn and revise before the GCSE exams. For parents the best way to support your child to understand and learn how osmosis works is by ensuring they understand what is happening in each of the three concentrations as they can then be applied to any example given.
We're confident if you follow this step by step approach your child will:
1) Understand the basic principles of osmosis and will be able to define it
2) Apply this to all three potential examples of osmosis in living organisms
3) Explain all three examples for both animal and plant cells back to you in detail
Step 1: Understanding the Scientific Terminology
Before we look at the process of osmosis, we must first understand some key scientific terminology. These terms will be constantly reinforced throughout this article, so it's useful to be able to recall them all.
A partially permeable membrane is one that allows only certain molecules to pass through. Often small molecules like water can pass freely across a membrane but larger molecules like glucose cannot.
If two solutions have the same concentration, we call this isotonic.
The solution that is more concentrated (has more dissolved solute and less water) is called hypertonic.
The solution that is more dilute (has less dissolved solute and has more water) is called hypotonic.
A solute is a dissolved substance such as glucose.
Step 2: Prior Knowledge Required...
All cells including animal, plant and bacterial cells have a cell membrane. The function of the cell membrane is to control the passage of materials into and out of the cell from their environment. Cell membranes are partially permeable, however, water, as it's essential for life is free to cross the membrane.
Animal and plant cells both have a cell membrane but only plant cells have a cell wall. The cell wall is made of a strong substance called cellulose which gives the cell structure and strength and prevents it from bursting if the plant cell becomes full of water (turgid).
Cells exchange materials such as oxygen through a passive process called diffusion. Diffusion is the movement of particles from a higher concentration to a lower concentration and uses no energy.
Step 3: So, What's Osmosis?
Osmosis is a special type of diffusion that involves water molecules only. Students must learn the definition for osmosis and then apply it to the following six examples.
Osmosis is the overall movement of water molecules from an area of higher water concentration to an area of lower water concentration through a partially permeable membrane.
Students need to be able to understand what is happening to osmosis in the three different concentrations.
Concentration 1: In a hypotonic solution
When a cell is placed in a hypotonic solution osmosis will occur.
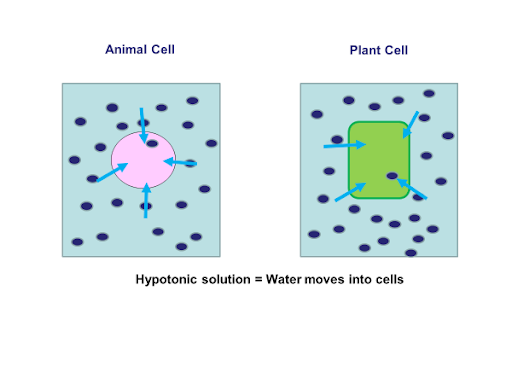
As you can see in the above diagrams both cells are in a hypotonic solution this means there are more water molecules in the solution than in the cells. Students would have to explain this by rewriting the osmosis definition and applying it to the example as follows:
Water molecules are moving into the cell via osmosis. They are moving from an area of higher water concentration (the solution) to a lower water concentration (the cell cytoplasm). Water is passing through a partially permeable membrane.
Students will need to know how will this affect each cell type. So, here we go...
Both cells would increase in mass due to the extra water uptake. The animal cell will eventually burst due to the pressure caused by the intake of water. The plant cell, however, will fill until no more water can fit into the cell which is called being turgid. The plant cell will not burst however because of the strong cell wall made out of cellulose covering its external surfaces.
Concentration 2: In an isotonic solution
When a cell is placed in an isotonic solution osmosis will not occur.
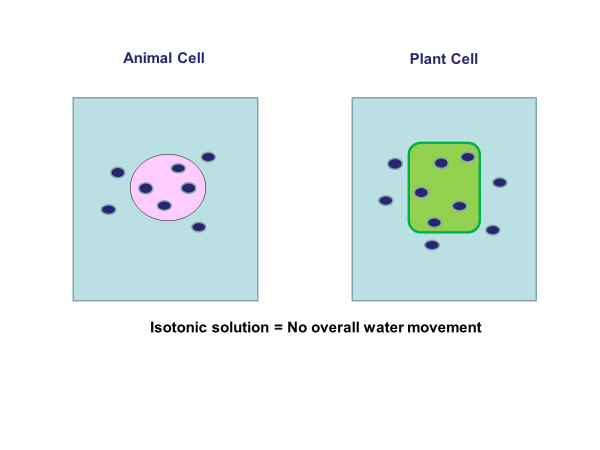
As you can see in the above diagrams both cells are in an isotonic solution. This means there is the same concentration of water molecules in the solution and in the cells. Students would have to explain this by rewriting the osmosis definition and applying it to the example as follows:
There is no overall movement of water molecules in or out of the cell via osmosis. This is because water moves from an area of higher water concentration to an area of lower water concentration but in this example, both concentrations are the same.
Students will also need to know how this will affect each cell...
Nothing would happen to either cell. Their masses would not change.
Concentration 3: In a hypertonic solution
When a cell is placed in a hypertonic solution osmosis will occur out of the cell.
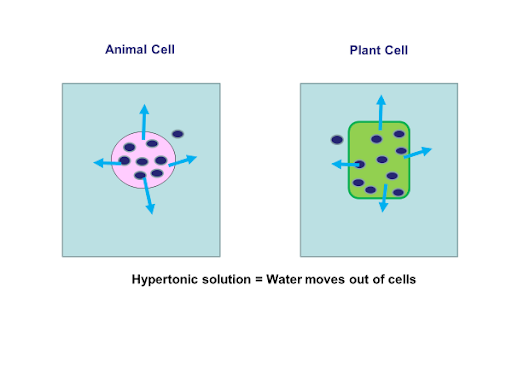
As you can see in the above diagrams both cells are in a hypertonic solution this means there are more water molecules in the cells than the solution. Students would have to explain this by rewriting the osmosis definition and applying it to the example as follows:
Water molecules are moving out of the cell via osmosis. They are moving from an area of higher water concentration (the cell cytoplasm) to a lower water concentration (the solution). Water is passing through a partially permeable membrane.
This affects each cell in the following ways...
Both cells would decrease in mass due to the water loss. The animal cell will shrivel up due to the loss of water. The plant cell vacuole and cytoplasm shrink and pull away from the cell wall. The cell wall would not move due to its strong structure. The cell undergoes plasmolysis.
Step 4: Give it a go!
Why not apply the above to the following example?
-
Write a definition for osmosis (3 marks)
-
Why does an animal cell burst when placed in a hypotonic solution, but a plant cell does not? (4 marks)
-
Why does osmosis not occur in an isotonic solution? (2 marks)
Stretch and challenge:
-
A potato chip is placed into a 5M sucrose solution. Explain why it loses mass.
-
How would the potato cells in the sample look different to blood cells put in the same sucrose solution if viewed under a microscope?
Step 5 - Putting it into Practise
Now, you’ve covered this together why not put this to the test and assign your child the following 5 activities in this order.
All activities are created by teachers and automatically marked. Plus, with an EdPlace subscription, we can automatically progress your child at a level that's right for them. Sending you progress reports along the way so you can track and measure progress, together - brilliant!
Activity 1 - Revise diffusion to support your understanding of osmosis
Activity 2 - Learn about and compare diffusion, osmosis and active transport
Activity 4 - Apply the Process of Osmosis to Cells
Activity 5 - Interpret Data Relating to Osmosis
Answers
a) Osmosis is the overall movement of water particles (1 mark)
From a higher water concentration to a lower water concentration (1 mark)
Through a partially permeable membrane (1 mark)
b)When placed in a hypotonic solution water enters both cells via osmosis (1 mark)
Water moves from a higher water concentration (the solution) to a lower water concentration (the cell cytoplasm) (1 mark)
Water moves across a partially permeable membrane (1 mark)
The animal cell bursts when the pressure inside the cell becomes too high, but the plant cell does not due to the strong cellulose cell wall (1 mark).
c) Osmosis is the movement of water from a higher concentration to a lower concentration (1 mark).
In a hypertonic solution, the concentration is the same as the cell so there is no overall movement of water.
Stretch and challenge:
d) A 5 M concentration sucrose solution would have a lot of solute in very little water so is a hypertonic solution.
Water molecules would move out of the cells via osmosis
From an area of higher water concentration (the cell cytoplasm) to an area of lower water concentration (5 M sucrose solution)
Through a partially permeable membrane.
e) Both cells would lose mass but under a microscope, the red blood cell (animal cell) would look shrivelled up but the plant cell would look the normal size because the cell wall does not change.
Keep going! Looking for more activities, different subjects or year groups?
Click the button below to view the EdPlace English, maths, science and 11+ activity library








