Think about the different liquids in your home. They have many different properties.
Liquids can be colourless, or coloured:


Odourless or smelly:

Transparent or opaque:

And very runny or really gloopy:
Liquids, like water, are also good at dissolving some solids in them.

What happens if you stir some of this sugar into the water?
It seems to disappear, doesn't it? But you know, really, that it's dissolved: it's still there, in the water, but in such tiny particles that you can't see them.
How can you prove that? Evaporate the water away and - hey presto! - there's the sugar.
But, what happens if we heat a liquid up ... or really cool it down?
Depends on the liquid, but if it's cooled past its freezing point, it generally changes into a solid.
We all know water, so let's consider a different liquid: the metal, mercury:

If you cool mercury down past minus 40oC, it freezes into solid mercury!
OK, let's consider another liquid: pure alcohol.

Looks like water, but it smells different and, if you heat it gently, it boils at just under 80oC (unlike water, which you probably know boils at 100oC.)
You see, each substance is made of particles (which are atoms or molecules) and these move (or don't!) according to how warm the substance is:
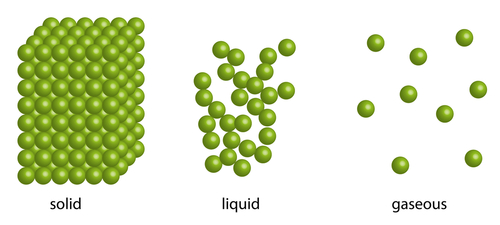
Take mercury: in a normal room, those particles are moving over each other, so it's in the liquid state (see the picture).
Take heat away and, around -40oC, the particles are arranged into a regular pattern (can you see that in the picture?).
On the other hand, add enough heat to go past 350oC, and the mercury boils to form a gas called mercury vapour, with the particles whizzing all over the room (not good - it's poisonous!).
OK, well let's see what else we can find out about the properties of liquids.

Are you ready?