How do we know that a chemical reaction is happening?
We can look out for some signs:
Bubbles - these are a sign that a gas has been produced in a chemical reaction.
A colour change - this can be a sign that a new substance has been made.
A temperature change - this is a sign that energy has been taken in or given out from a chemical reaction as bonds are broken or formed.
We can measure how fast a chemical reaction is occuring by looking at these signs.
For example:
When iron fillings and powdered sulfur are mixed and then heated, a chemical reaction happens and iron sulfide is made. We know when the reaction has taken place because there is a colour change - from yellow to black. We can use a stop watch to time how long it takes the mixture to turn black. That is one way to measure how fast a reaction occurs.

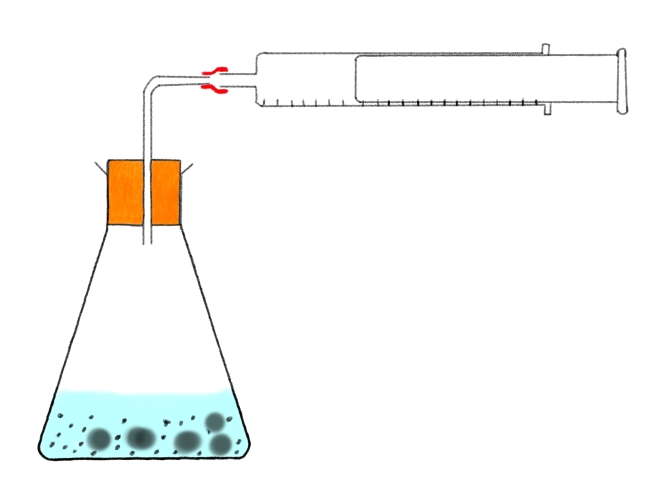
When vinegar and baking soda are mixed together, a chemical reaction takes place to make sodium acetate, water and carbon dioxide. We would see bubbles which would tell us that a reaction is happening. But we could see how fast it is happening by collecting the gas produced in a gas syringe and measuring how much gas is produced in a certain time.
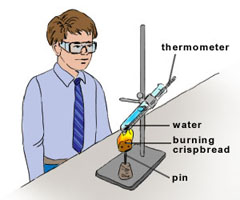
When a metal such as magnesium is dropped into a beaker of acid such as hydrochloric acid, a chemical reaction occurs. We know because there is a temperature change - the beaker gets hot. We could see how quickly the chemical reaction happens using a thermometer, by measuring the temperature change in a set time.
Let's try some questions on this now.








