Everything in the world is made from very small particles called atoms. If all the atoms in a substance are the same, then it can be classed as an element.
Elements contain only ONE type of atom.
There are over 100 elements known to us, and scientists are still working hard to try and discover new elements. All the known elements are arranged in the Periodic Table:
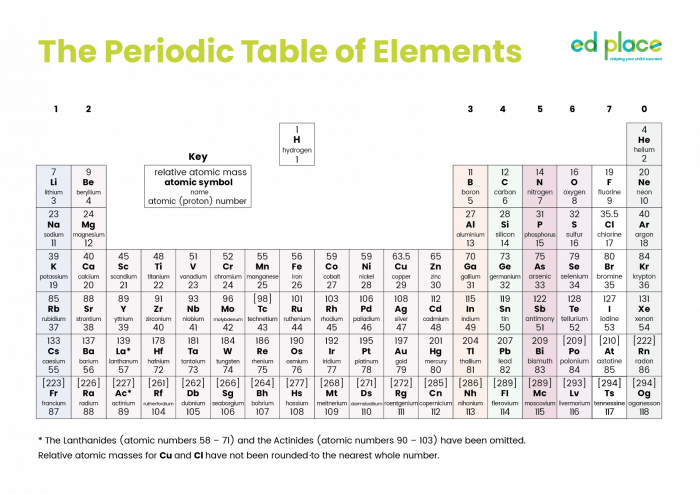
A Russian scientist named Dimitri Mendeleev developed the Periodic Table.

He realised that elements can be arranged in a regular pattern. He began to arrange the elements based on their atomic number, and group together elements which react in similar ways. This resulted in the Periodic Table, which can be found on the wall of almost every science lab in the world.
The Periodic Table lists all the elements in a specific order, to help us understand why elements react as they do and what properties they might have.
The Periodic Table is so well organised that all the metal elements are on the left-hand side of the table and all the non-metals are on the right:
.jpg)
Let's try some questions now.








