Atoms are the building blocks of everything. You can think of them as lego bricks. They can exist on their own or be combined in lots of different ways, either with other colours like them or with different colours. However, unlike lego bricks, atoms are super-small, and can't be seen with the naked eye.

They have a radius of about 0.1 nanometre! If people were the size of an atom, the entire population of the world would fit into a box about 1,000th of a millimetre across.
Because we can't see them, we show atoms as small coloured circles. Atoms of the same element are the same colour and size. Different types of atoms would be shown as different coloured circles.
Atoms can exist on their own or joined to other atoms of the same type to make up an element, or can form bonds with other atoms to form molecules.
For example, carbon atoms can be joined to other carbon atoms to form super strong diamond, or joined to oxygen to make carbon dioxide gas, or even joined with oxygen and hydrogen to make a liquid, alcohol. No matter what, the atoms of carbon remain the same. They cannot be destroyed or changed themselves, they remain the same size, it's just their arrangement that can change.
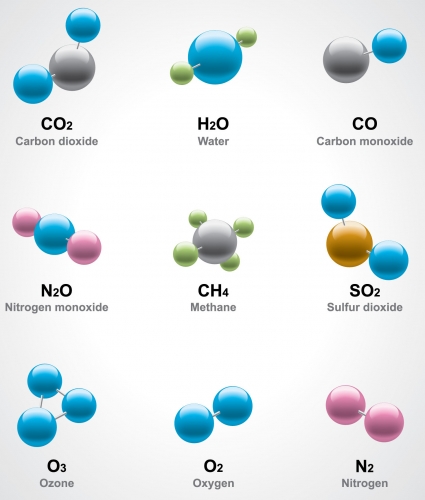
The image above shows various molecules. The circles represent individual atoms.
We can see that some molecules are made from only one type of atom, such as oxygen and ozone. But some are made of two or more different types of atom, such as methane or nitrogen dioxide. We can sometimes tell how many atoms are in a molecule by looking at the name of that molecule. If the name of an element has a 'di' before it, it means '2 atoms of that element', if it has 'mon' before it, it means '1 atom of that element'.
So if you look at the molecules above you can see that carbon dioxide has 1 carbon atom and 2 oxygen atoms because it says 'di-oxide' which means 2 oxygen atoms. In carbon monoxide, you can see that there is 1 carbon atom and 1 oxygen atom as it says 'mon-oxide' which means 1 oxygen atom.
Ready for some questions on these little things we call atoms? Great, let's go........