This is the Periodic Table of all the elements known so far (click on the image to see a larger version):
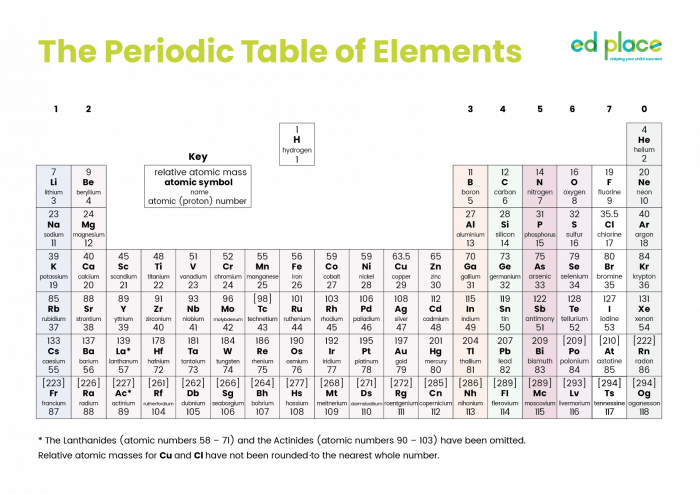
The Periodic Table was constructed after consideration of the physical and chemical properties of the elements. It is possible to predict the properties according to the location of each element. The horizontal rows are called periods and the vertical columns are called groups. Properties of elements change across a period and down a group.
Physical properties are measurable values that describe the physical state of an element, for example, whether it is a solid, liquid or gas at room temperature.
Chemical properties however are qualities of the element evident in a chemical reaction, for example, all Group 7 elements react with metals to produce a salt.
Certain elements in a group may share physical properties, for example, the elements in Group 0 all have low boiling points.
Group 0 elements don't take part in chemical reactions as they are very unreactive - this is a chemical property that these elements share.
Generally, elements are solid on the left-hand side of the table and change to liquid and then to gas as we move to the right. There are some exceptions to this rule, for example, hydrogen is on the left, but it is naturally a gas.
Melting and boiling points also show trends across a period, but the trends are a bit more complicated. Generally, from left to right the melting and boiling points rise and reach a peak, after which they fall again - still moving to the right of the same period.
Metals are towards the left and centre of the Periodic Table and non-metals are on the right of the table.
As we move from non-metals to metals (right to left), the elements become better conductors of electricity and heat, more malleable (can be reshaped easily) and ductile (can be stretched into wires).
You can see pictures of a metal and a non-metal, respectively, below:


That's a lot of information to take on board but let's give the questions a go!
You can check back to this introduction at any point during the activity by clicking on the red Help button on the side of the screen.







