Everything in the world is made of atoms.
That means it's important for scientists to try to understand exactly what atoms themselves are made of.
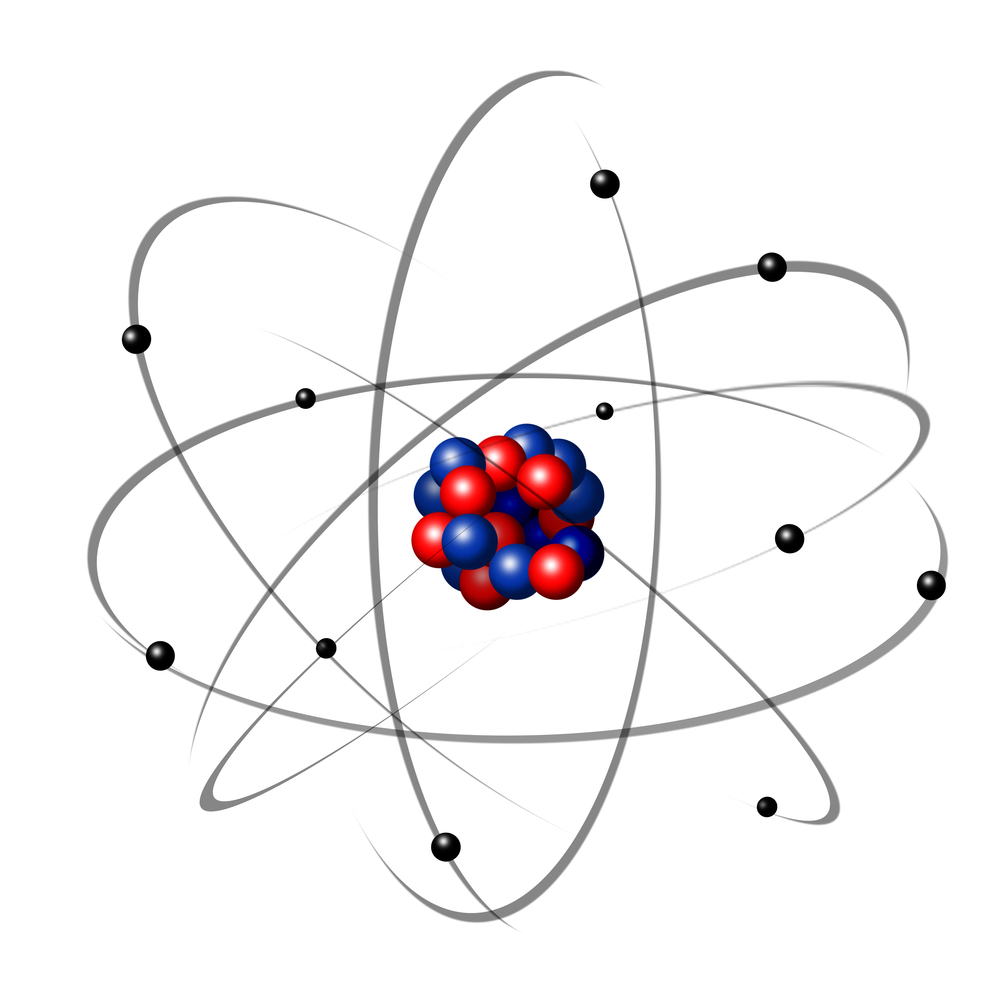
Atoms are made from three smaller subatomic particles:
Electrons - are very light particles that orbit around the nucleus at the centre of the atom, in layers called energy levels or shells. The electrons are the black particles in the diagram above.
Protons - are found inside the nucleus of the atom. The protons are the red particles shown in the diagram. The nucleus is the centre of the atom.
Neutrons - are also found inside the nucleus of the atom. The neutrons are shown by the blue colour in the diagram above.
There's lots more to discover about atoms but these questions are an excellent place to start your journey!
Are you ready to begin?








