Pure substances have a sharp melting point, which means they melt at a fixed temperature. We can show this on a graph called a cooling curve.
A cooling curve is produced by measuring the temperature of a substance as it cools and changes state from liquid to solid. The point at which it freezes can be identified from the graph. The flat section on the graph shows the freezing point.
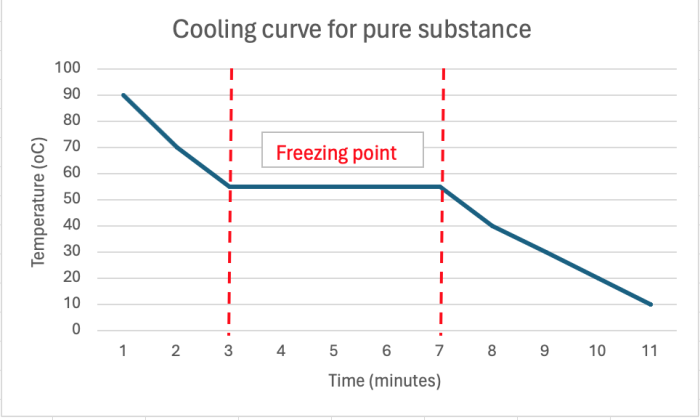
Having a flat line on the graph shows us that the freezing point is exact - for this substance the freezing point would be 55oC. Another term for freezing point is melting point. So the melting point for this substance will be 55oC too.
Let's try some questions on this now.







