If you were asked the question, "What would happen if you placed a metal into water?" you might expect the answer to be "Nothing" or "It would sink".
Normally, your answer would be correct because most metals do not react with water and are quite dense, and so would sink. But as always in chemistry, there are some exceptions to the rules - the alkali metals.
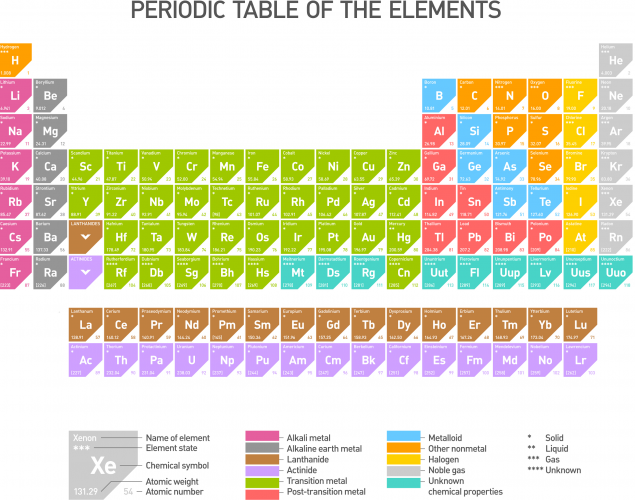
The alkali metals are found in Group 1 of the Periodic Table (the pink column in the Periodic Table below). All the alkali metals are stored in oil or an unreactive gas such as argon to prevent them from reacting with the moisture in the air.
They react very strongly with water to produce hydrogen gas and an alkali (metal hydroxide). This is shown in the equation below:
alkali metal + water![]() metal hydroxide + hydrogen
metal hydroxide + hydrogen
This reaction is very vigorous and usually involves lots of smoke, flames and can even involve explosions. The metals become more reactive as you travel down Group 1, so we will start at the top of the group with lithium.
Lithium
Lithium is the least reactive alkali metal, but still reacts to produce lots of smoke and heat. This can be seen from the picture below:
.png)
Note how the lithium floats on top of the water because it has a low density.
Sodium
Sodium is more reactive than lithium and will produce yellow flames when placed in water.

Potassium
Potassium is the most reactive metal that is allowed in the school classroom.
It is very reactive and burns with a lilac flame when placed in water. It may also cause an explosion of sparks, which is very spectacular!
.png)
Rubidium
Rubidium is not allowed in the school lab because it explodes when placed in water.
It sinks to the bottom slowly because it has a higher density than potassium and then explodes.
.png)
Caesium
Caesium is again more reactive and will explode with a lot of force if placed in water.
.png)
Francium
Finally, we have francium, which is very reactive and very rare.
It is estimated that only 30 g of francium exists on the entire planet, so we can't see how it would react with water.
Let's have a go at some questions now.