Conservation of mass
When chemicals react, the mass of the reactants is always equal to the mass of the products. The total mass in a reaction never changes. If in an exam question, it is given that the mass has decreased, it will be because a gas was produced and it escaped. If it is given that the mass has increased, the reason will be that a gas, like oxygen, has reacted with the chemical.
Example question: When calcium carbonate is heated, it decomposes to make calcium oxide and carbon dioxide. 20 g of calcium carbonate produced 11.2 g of calcium oxide. How much carbon dioxide was made?
Answer: 20 g of reactant must end with 20 g of product. We know that the products are calcium oxide and carbon dioxide, so:
20 (g of calcium carbonate) - 11.2 (g of calcium oxide) = 8.8 g of carbon dioxide were produced.
Using simple ratio
When we know reactant and product mass for a reaction, we can use it to calculate different masses for the same reaction.
Example question: 3 g of magnesium react with oxygen to give 4.6 g of magnesium oxide. How much magnesium oxide will 6 g of magnesium give?
Answer: The given mass is 3 g and the question asks about double the mass, i.e. 6 g. Double the amount of magnesium will give double the amount of magnesium oxide; so, 4.6 x 2 = 9.2 g of magnesium oxide will be produced.
Extension question: How much oxygen was used in the reaction if 6 g of magnesium produced 9.2 g magnesium oxide?
Answer: 9.2 - 6 = 3.2 g of oxygen reacted with magnesium.
Yield and percentage yield of a reaction
The yield of a reaction is the amount of chemical that was produced in the reaction. The more reactant used, the more product is given. 100% yield means no product has been lost, whereas 0% yield means no product was made.
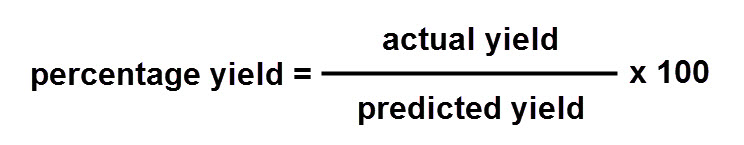
To calculate percentage yield, we use the following formula:
Relative atomic mass
Atoms of different elements have different masses. The carbon card below is from the periodic table. Did you notice number 12 on the bottom? This is the relative atomic mass of carbon. Different periodic tables have them in different positions, but always look for the larger number between the two.
.jpg)
Relative formula mass
To calculate the relative formula mass of a compound, simply add up all the numbers of the elements in a formula.
Example question: Calculate the relative formula mass of water. The formula for water is H2O.
Answer: In an exam, you may be given the relative atomic masses of hydrogen (H) and oxygen (O) directly but you may just be given an extract from the periodic table and be expected to find them yourself. The relative atomic mass of hydrogen is 1 and for oxygen it is 16. Water has two atoms of hydrogen (H2) and one atom of oxygen (O), so:
relative formula mass of water = 1 + 1 + 16 = 18
Have another look at the information in this Introduction before moving on to the questions, just so you can be sure you have got your head around it all.








