It's a classic scenario - you are trapped on a desert island with no hope of rescue and the only thing to drink is water that has a load of salt and sand in it. How do you get clean and pure drinking water? You use some of these separation techniques - that's how. Remember these, they might save your life if you get trapped with scientific equipment on a desert island with no hope of rescue! It's a common occurrence - honest!
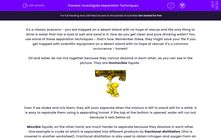
Oil and water do not mix together because they cannot dissolve in each other, as you can see in the picture. They are immiscible liquids.
Even if we shake and mix them, they will soon separate when the mixture is left to stand still for a while. It is easy to separate them using a separating funnel. If the tap at the bottom is opened, water will run out because it sets below oil.
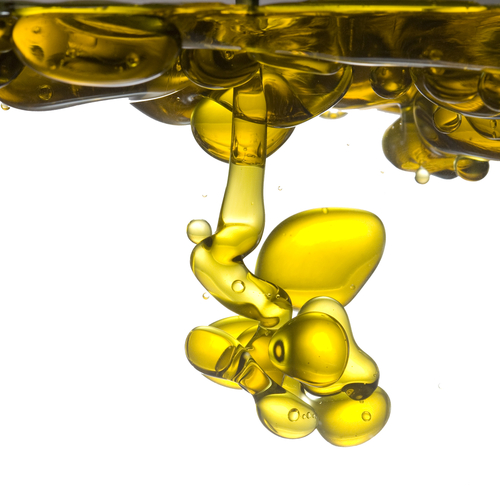
Miscible liquids, on the other hand, are much harder to separate because they dissolve in each other. One example is crude oil which is separated into different products by fractional distillation (this is covered in another worksheet). Fractional distillation is also used to obtain nitrogen and oxygen from air. The diagram below illustrates how it happens. Air is filtered to remove dust and then cooled to -200°C. As the air is liquefied, water vapour and carbon dioxide are removed and oxygen and nitrogen are collected from separate exits at the temperatures shown in the diagram.
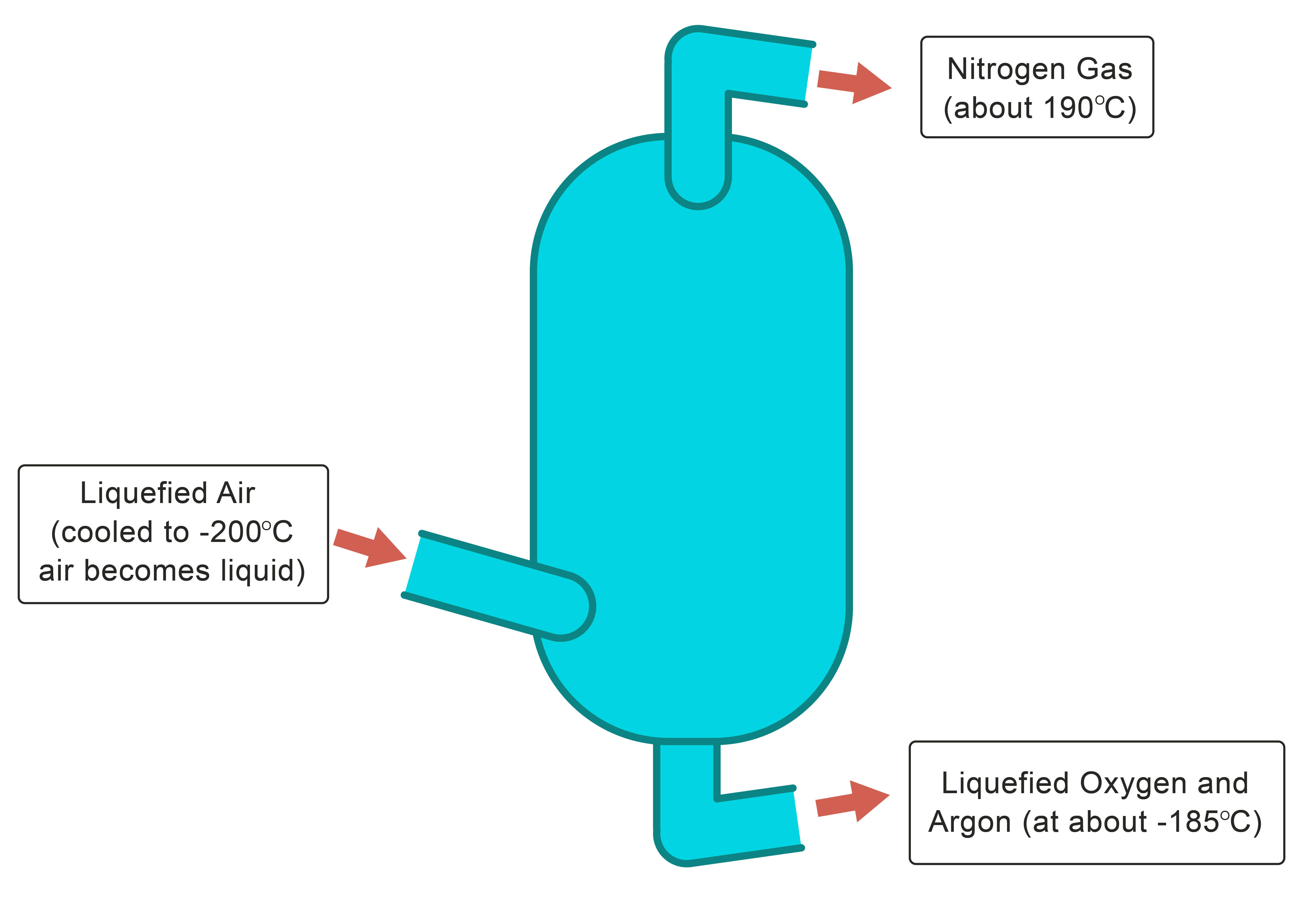
Chromatography is another technique which is used to separate mixtures of colour compounds like inks and dyes. A spot of the mixture is placed on a special piece of paper (see diagram below) and it is allowed to soak up a solvent. As it soaks up, the solvent separates the different components because they travel different distances at the same time.
Components have set Rf values when chromatography is conducted in exactly the same way, so the substances can be identified by scientists. It is calculated with this formula:
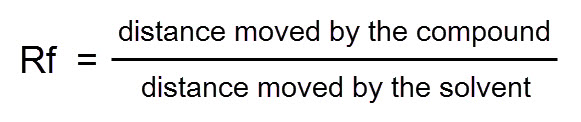
Are you ready to try some questions now?