During chemical reactions, energy is transferred to and from the surroundings. This transfer is in the form of thermal energy, i.e. heat. A chemical reaction can be either exothermic or endothermic.
Exothermic Reactions
Exo means 'out', so exothermic reactions release heat to their surroundings. The picture shows an example of an exothermic reaction:

Clearly, this looks like an explosion. Explosions are exothermic reactions, but a reaction can be exothermic without exploding - any increase in temperature indicates an exothermic reaction. If you were to hold a container when an exothermic reaction is taking place, you would feel the heat and a thermometer inside the container would show an increase in temperature. Examples of exothermic reactions are combustion (burning) and neutralisation.
Endothermic Reactions
Endo means 'inside', so endothermic reactions take in energy from their surroundings. Be careful because this can be confusing: if you were to hold a container when an endothermic reaction is taking place, you would feel your hands getting cold as the reaction would take heat from the container and even your hands, if you kept holding it! A thermometer in the container would show a decrease in temperature.
The graph below shows the difference in energy levels of reactants and products in an endothermic reaction:
.png)
As you can see, the energy of the products is far higher than the energy of the reactants. Examples of endothermic reactions are electrolysis and thermal decomposition.
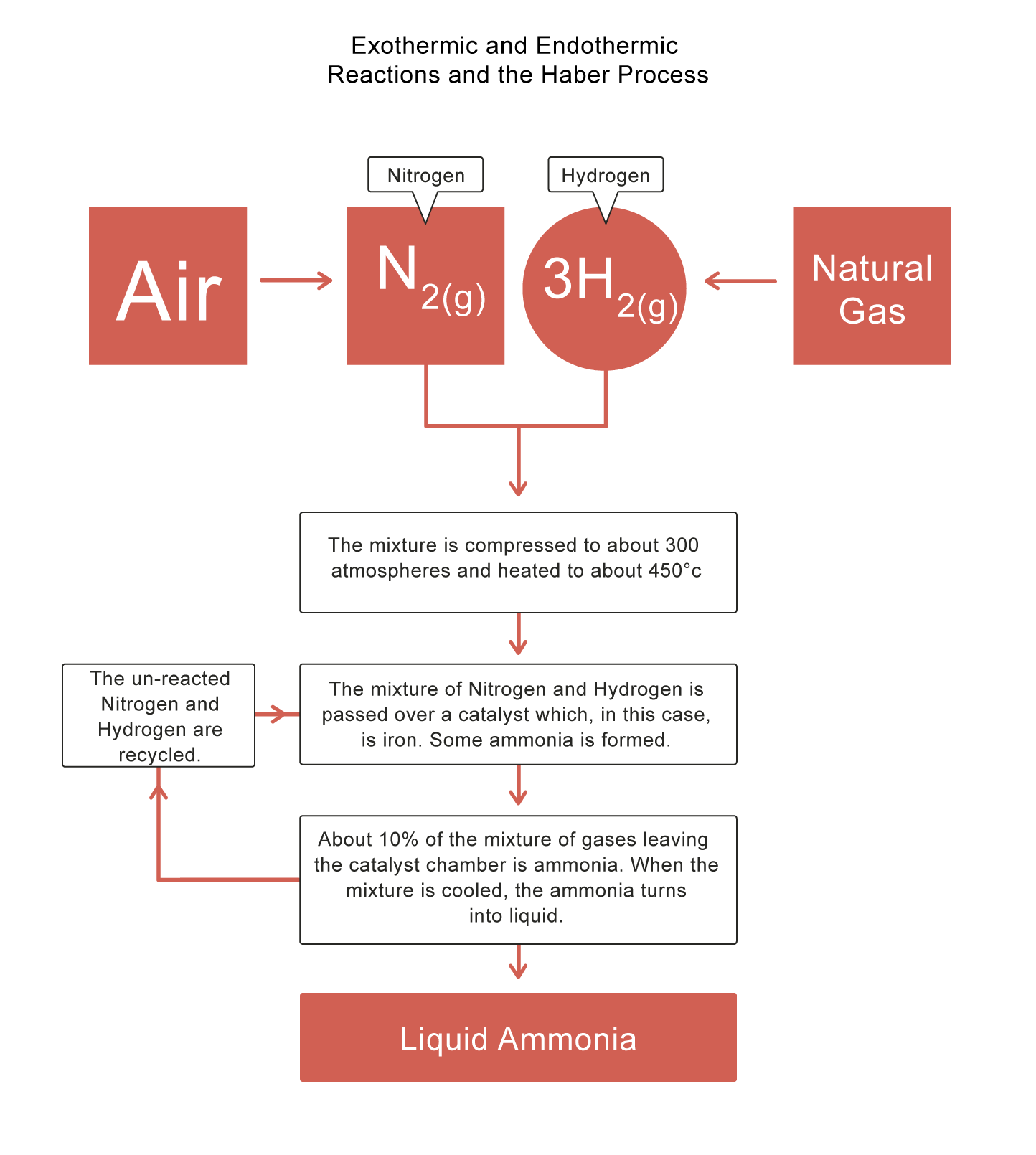
The Haber Process
Some reactions are reversible, which means the products can react to produce the reactants again! The Haber Process is an example - it is used in industry to produce ammonia - a compound of nitrogen and hydrogen. Ammonia is a colourless gas used in the production of fertilisers, nylon, dyes, cleaning products and explosives.
The chemical equation below shows the Haber Process (note that a double arrow is used to show reversibility):
.png)
The forward reaction in the Haber Process is exothermic, i.e. when ammonia is produced, heat is released. This means an increase in temperature shifts the equilibrium to the reverse reaction, so less ammonia is produced. However, if the temperature is too low, not enough ammonia will be produced.
The Haber Process is illustrated in the diagram below:
Time for some questions now!








