Suppose that we have a chemical reaction that we would like to happen faster. For example, if we could speed up the reactions in fireworks, the bang would be louder, the sparks would move faster and be brighter. It would be great.

Imagine running a factory that makes chemicals for medicines. If we could make the chemicals we needed more quickly, we could sell them faster and more profitably (time is money).
On the other hand, some chemical reactions are harmful - such as the chemical weathering of buildings and statues.
Ideally, we want harmful chemical reactions to happen as slowly as possible. Either way, it is useful to control the rate of a chemical reaction.
There are five ways that we can speed up (or slow down) a chemical reaction, all linked by collision theory:
For two particles to react, they have to collide in the right orientation and with enough energy to break the atomic bonds in the particles.
The more frequently these collisions happen, the faster the rate of reaction will be.
From this, we get five factors which affect the rate of a reaction:
Temperature
Concentration
Pressure
Surface area
Catalysts
All of these factors affect the reaction rate by changing the frequency of collisions between reactant particles, or changing the likelihood that a collision will be successful (right orientation and enough energy).
As well as learning the five factors, think about the connections to collision theory.
Temperature
When the temperature increases, the particles have more energy. More energy means more movement, so the frequency of collisions increases.
Also, the collisions that happen have more energy, so are more likely to cause bond-breaking. Both these factors increase the rate of reaction.
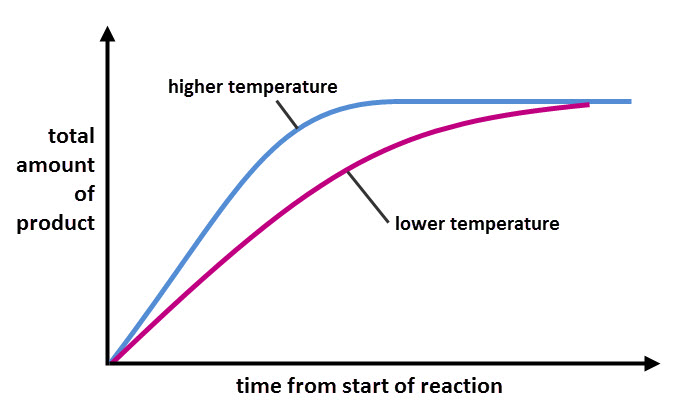
The graph below shows the rate of the same reaction in different temperatures. The blue line shows the progress of the reaction at a higher temperature than the purple line. The blue line is steeper, showing that the reaction happens faster.
Notice that the final amount of product made is the same for the two temperatures. That's because the amount of product made depends on the amount of reactant used, not the rate of reaction.
Concentration
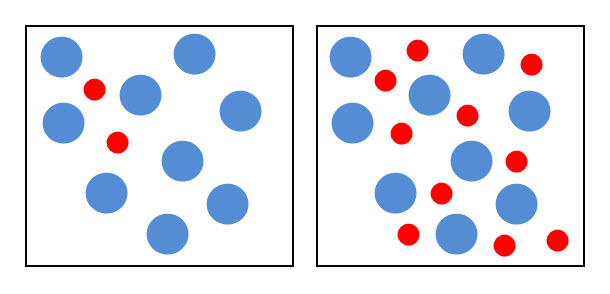
If the concentration of one or more of the reactants increases, the particles become more crowded. The diagram below shows the particles of two chemicals. The box on the right contains more red particles representing one of the chemicals about to react. This means that the concentration of the 'red' chemical is now higher, but the particles are still in the same space. This increases the probability of collisions and so the rate of the reaction.
Pressure (in gases)
An increase in pressure speeds up a reaction. It has the same effect as an increase in concentration. The way you increase pressure on a gas is by squeezing it into a smaller volume, but the mass remains the same. This results in the same number of particles moving about in a smaller volume, which increases the number of collisions and the rate of the reaction.
Surface area
When a solid chemical is broken down into smaller pieces - or even a powder - there are more particles that can react, as the diagram below shows.
We say that a chemical in a powder form has more surface area than the same mass of the same chemical in a solid block form. An increased surface area allows for more collisions and the rate of the reaction increases.
One example of this is that custard powder is very explosive because the powder has very small grains.
.jpg) |
.jpg) |
Catalysts
A catalyst is a chemical that speeds up a reaction without being used in it. Catalysts are specific to reactions, so the catalyst for one reaction would not work for another.
In biology, catalysts are called enzymes - it's a different name for the same idea.
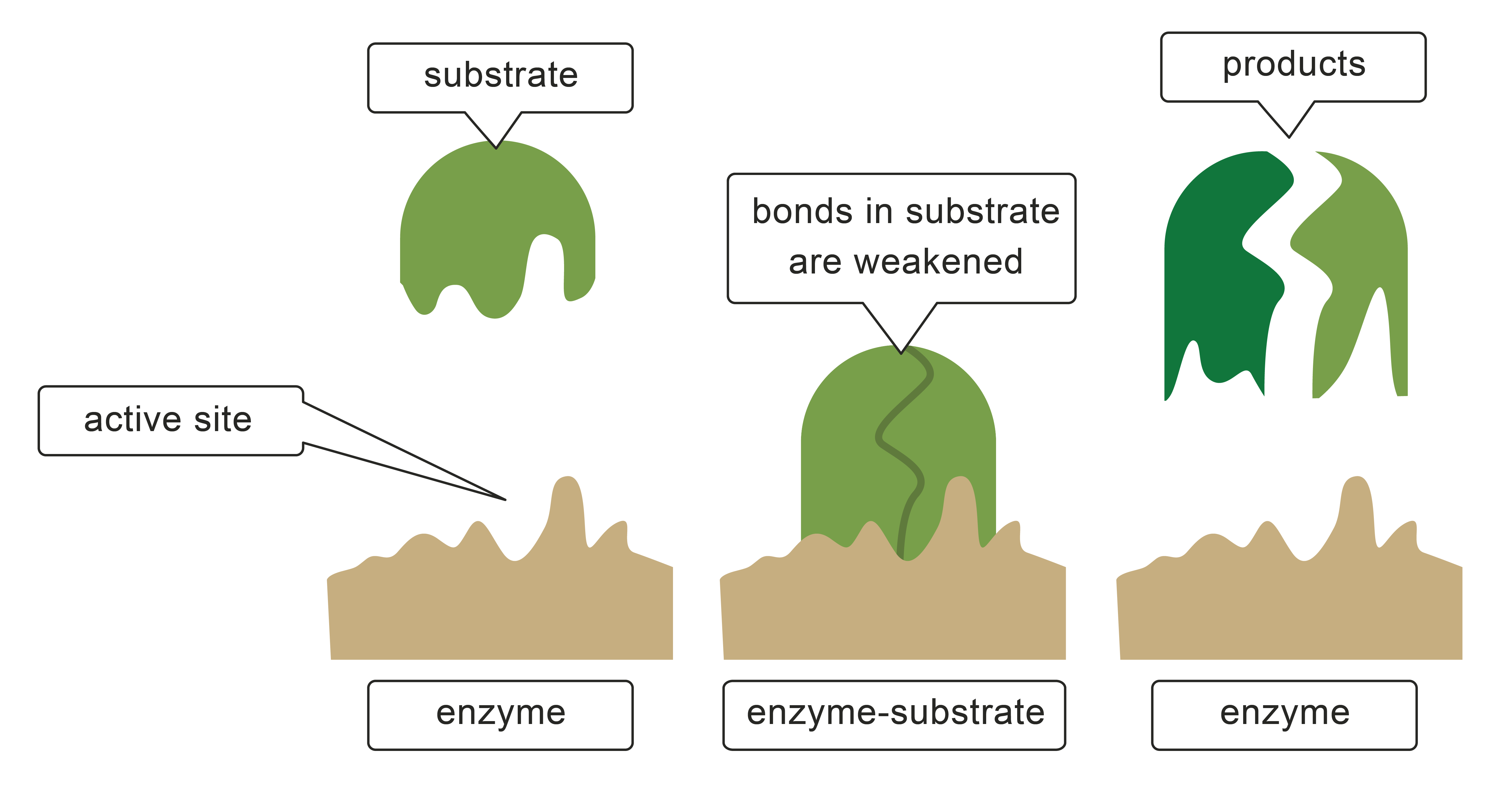
Catalysts don't increase the frequency of collisions, but they do make collisions more likely to be successful.
In the diagram, the catalyst is the brown shape at the bottom of the picture. It holds the reactants in specific positions and orientations, which are the correct ones for a reaction to happen. The catalyst (or enzyme) can also make chemical bonds to the reactants which weaken some of the bonds which need to be broken for a reaction to take place.
If less energy is needed to turn a collision into a reaction, there is a higher chance that a collision will cause a reaction. In terms of energy level diagrams, we say that the catalyst has reduced the activation energy.
If we want a reaction to go more quickly, we can try these things:
Increasing the temperature.
Increasing the concentration of the reactants in solution.
Increasing the pressure of the reactants, if they are a gas.
Increasing the surface area, by grinding the solid into smaller pieces.
Using a catalyst (called an enzyme in biology).
If we want a reaction to go more slowly, we can try the opposite of these things.
Are you ready to have a go at some questions?








