When substances are mixed together some mix easily, others refuse to mix - how can you tell?
Can they be separated from each other - and if so, how?
Can the mixing be reversed?

You'll have tried some of these out - salt or sugar added to water, for example. These dissolve in the water to form a solution - like salt solution.
Can you get them apart again? What about filtering?
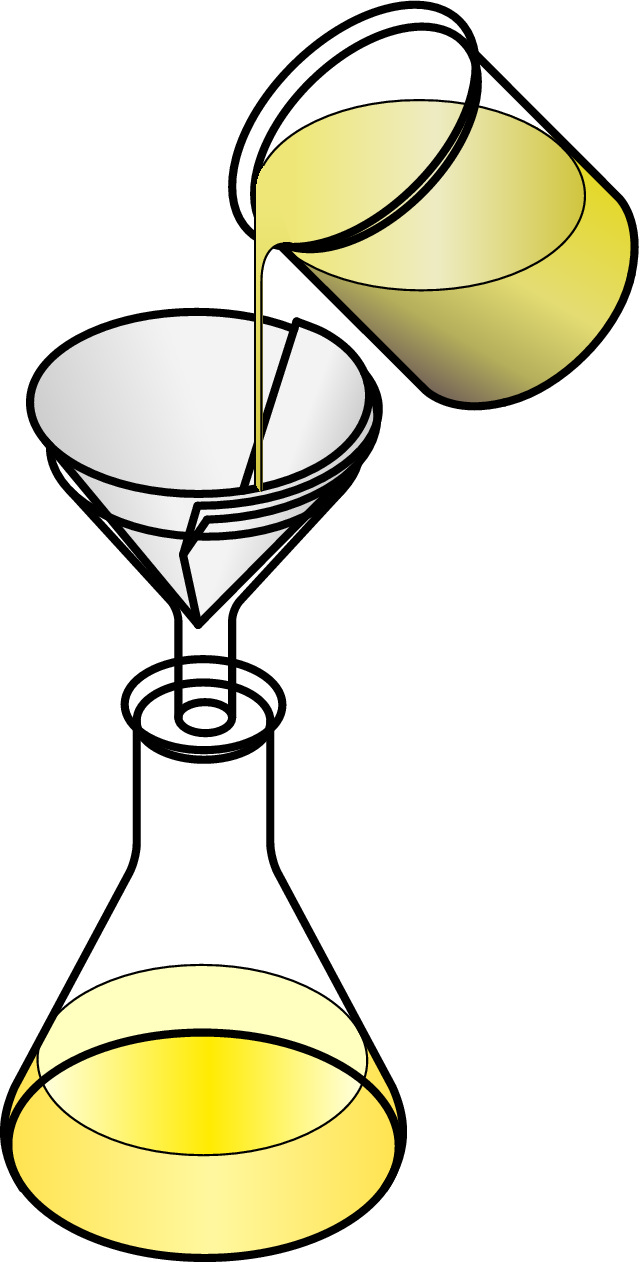
The dissolved salt is too small to be trapped in the filter paper, isn't it?
Maybe evaporation would work:
.png)
Now the water boils away, evaporating to leave the salt behind.
So, dissolving is a reversible change - you can get back what you started with.
What about non-reversible changes - what sort of changes are these?
Well, these are the sorts of questions that you'll be able to explore as you follow this activity through.
Let's get started.