In this activity, we're going to take a journey to the seaside with Amy and Sam to investigate seawater: what it's made of and how to separate its various constituents.

A bucketful of seawater contains lots of things: water, salt, seaweed and sand, just for starters!
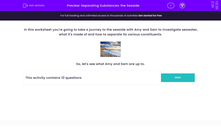
Think: how can we separate the big bits, like seaweed?
The easiest way is to use a filter. A filter works like a fine sieve, trapping bits (like seaweed) that are larger than the tiny holes in the filter paper.
Think: how can we separate the salt from the seawater?
If we evaporate the water away, by heating the seawater, then the salt is left behind. The salt is a dissolved solid in the seawater and only the liquid water evaporates away.
Think: how can we get drinking water from seawater?
If we boil the seawater, the water evaporates away as steam. If we can collect the steam and cool it down, it condenses back into pure water that's safe to drink.

See if you can use these ideas to help Amy and Sam as they investigate seawater.