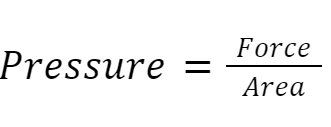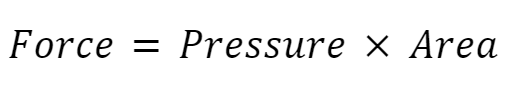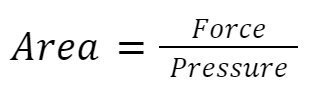All around you right now, gas particles are moving and colliding with nearby surfaces. Gas particles are spread out, and they move in random directions.
When gas particles collide with surfaces, they cause gas pressure. Let's learn about some factors that affect pressure, and make sure we know how to calculate that pressure!
Imagine a blown-up balloon. That balloon has air particles inside. They move in random directions, colliding with the inner walls of the balloon, maintaining its shape.
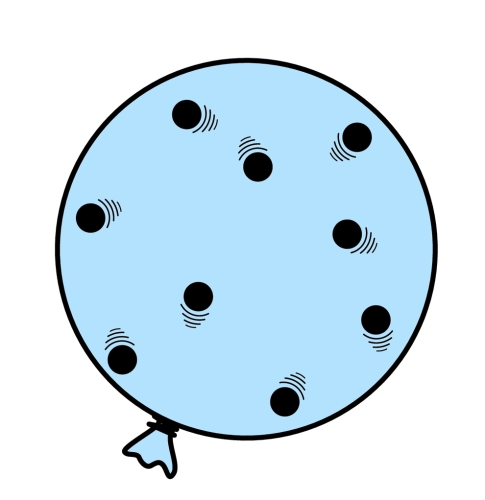
When the air particles collide with the wall of the balloon, they cause gas pressure. We can calculate the pressure like this:
Force is measured in Newtons (N), area is measured in metres squared (m2), and pressure is measured in Pascals (Pa) or Newtons per metre squared (N/m2)
We can increase or decrease gas pressure in a few ways.
1. Temperature affects gas pressure.
If you increase the temperature of a gas, you give the gas particles more heat energy. This heat energy makes the air particles move faster.
The faster the particles move, the more frequently they collide with the walls of the container they are in. That means they provide a bigger force. That bigger force causes a bigger pressure.
If you cooled down a gas the opposite would happen! The particles would lose heat energy, move slower, collide with the walls of the container less, and cause less pressure as a result.
2. The number of particles affects gas pressure.
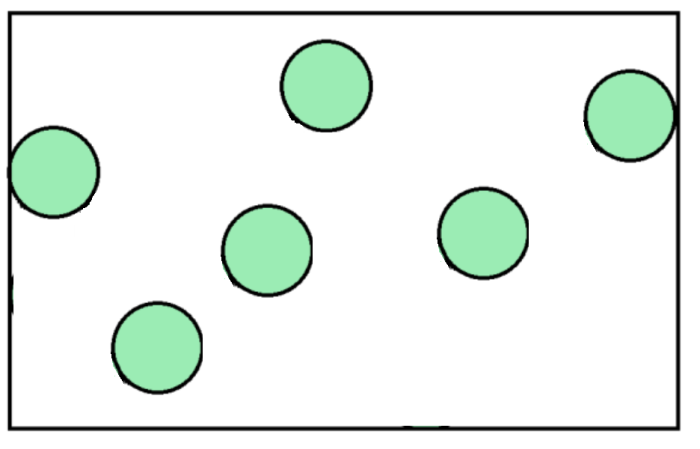
Look at these two diagrams. They show gas particles inside identical containers.

.png)
The second container has more gas particles. As a result, there will be more collisions between particles and the wall per second than the container with fewer particles. The more particles there are, the higher the pressure! If there are fewer particles, the pressure will be lower.
Similarly, if you took the same number of gas particles but put them in a smaller container with less volume, they would collide with the walls more frequently, increasing the pressure.
Let's practise using the pressure equation:
We can actually rearrange the equation like this:
Let's try using the rearranged equations!
Example
A tyre has an air pressure inside of 2,200 Pa. The internal area of the tyre is 0.6 m2
What is the force on the inside of the tyre, provided by the air particles?

Answer
First, let's decide which equation to use.
Force (N) = 2,200 Pa × 0.6 m2
Force = 1,320 N
Now let's check our understanding with some questions!