An atom is the smallest chemical entity. Its name comes from a Greek word that means 'a whole', 'a unit', 'something that cannot be broken'. Of course, scientists have now actually managed to separate atoms into their components, but they are still the smallest units that make up chemical elements.
An atom is made up of a nucleus and electrons that surround it. The nucleus carries a positive charge and the electrons a negative charge. The nucleus is made up of protons, that carry the positive charge, and neutrons, which carry no charge because they are neutral. You can see how atoms are represented in diagram form below. In reality, they are a bit more complicated! You can draw the electrons as dots or crosses.
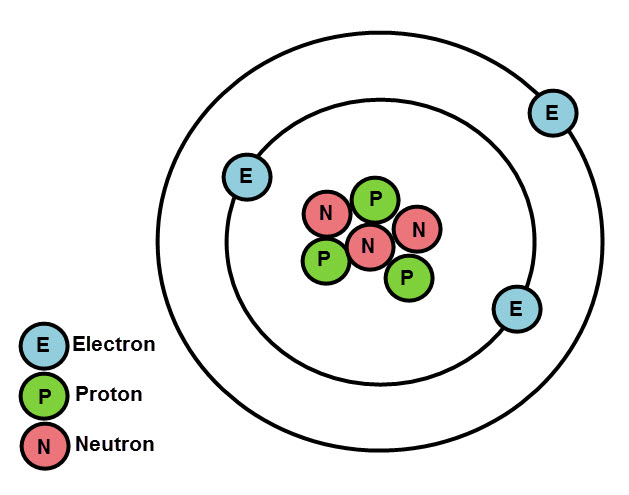
Each atom has an atomic number and this is written next to the symbol of the corresponding element in the periodic table. Yes, you guessed right: each element has its own atom, that's what makes each element different.
The atomic number is the number of protons in the nucleus. Usually, the number of protons is the same as the number of neutrons - but not always!. The number of protons is the same as the number of electrons.
Atoms also have a mass number. This is the total number of protons and neutrons. The particles of an atom also have a mass, but because it is tiny we call it relative mass, so protons and neutrons have a relative mass of 1 (no unit!), whereas electrons are so tiny that their mass does not even count.
Some atoms of the same element have the same atomic number, but a different mass number (due to the different number of neutrons). These are called isotopes.
Electrons are arranged around the nucleus in shells (different energy levels) shown in the diagram above. Suppose the atom shown is lithium - we would write it as Li 2,1. This means the lithium atom has 2 electrons on the first shell (inner shell, near the nucleus) and one on the outer shell. The first shell can have a maximum of 2 electrons, the second 8, the third 8 and the fourth 2.
The properties of the different elements depend on the number of protons and electrons they have. They are placed on the periodic table in groups in the order of their atomic number.
Let's move on to the questions now.








