Ionic and metallic bonding each only make one type of structure. Covalent bonding is a bit harder because there are two types of structure that form. Don't panic because you will soon understand both of them.
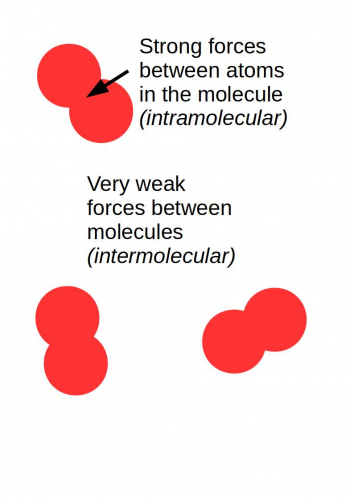
Most covalent compounds form simple structures, also called simple molecules, or simple molecular structures. Simple molecules are tiny - many of them only contain two or three atoms. The forces between the atoms in each molecule are made by covalent bonds, so they are very strong. Scientists call these intramolecular forces - 'intra' at the beginning of a word means inside.
The forces between separate molecules are very, very weak. Scientists call these forces intermolecular forces - 'inter' at the beginning of a word means between - think of international, or internet.
This means that the molecules in oxygen gas look like this:
The properties of simple covalent structures depend on the weak intermolecular forces - it doesn't really matter that the forces within the molecule are strong.
Simple covalent molecules:
Have very low melting and boiling points: Oxygen turns from a solid to a liquid at -219°C, and from a liquid to a gas at -183°C. Larger molecules have higher boiling points, but still lower than for ionic or metallic bonding.
Are usually gases or liquids at room temperature.
Dissolve easily in water
Do not conduct electricity because there are no mobile electrons or ions.
Although most covalent substances make simple molecules, a few make giant structures - more like the structures formed by metallic or ionic bonding. The important point about covalent giant structures is that all the bonds linking atoms are covalent, so all the bonds are very strong indeed.
Giant covalent structures are usually based on carbon or silicon. The reason for this is that they can make four covalent bonds per atom, which is more than most other elements. You need four covalent bonds to make a giant structure with bonds in three dimensions.
Diamond is a giant covalent structure made of carbon atoms:
(1).png)
This means that covalent giant structures:
Are very hard because the covalent bonds are very strong.
Have very high melting points because huge amounts of energy are needed to break covalent bonds.
Do not dissolve in water because water cannot weaken covalent bonds.
Do not conduct electricity because there are no mobile electrons or ions.
The key difference between molecules and giant structures is that giant structures only have covalent bonds. To make a liquid or solid from molecules, we need other (very weak) forces to hold the molecules together.
Now it's time for some questions.







