Niels Bohr worked out that electrons can only be in certain parts of the atom. In this activity, you will learn how to apply his rules, and understand how useful they are.
Bohr worked out that electrons move around in shells, around the nucleus. For example, a potassium atom is drawn like this:
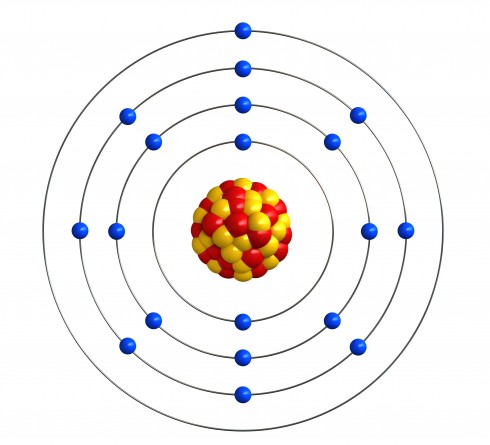
Potassium has the atomic number 19 and mass number 39, so there are 19 protons and 20 neutrons in the nucleus. As there are 19 protons, there must also be 19 electrons to make a neutral atom. They are spread across four shells, according to these rules:
The first shell, closest to the nucleus, can only take 2 electrons.
The second shell can only take the next 8 electrons.
The third shell takes the next 8 electrons.
The fourth shell takes the remaining electrons.
These rules only work exactly for the first twenty elements. For elements heavier than calcium, the rules get more complicated. You won't be asked to work out the electron structure for (say) iron (atomic number 26), but later on, you will find that iron has some odd properties because of the extra rules.
So, for potassium, we think about the electrons this way:
Potassium has the atomic number 19, so there are 19 electrons to put in place. The first two fill up the first shell, so there are 17 left. The next 8 fill up the second shell, so there are 9 left. The next 8 go into the third shell, so there's only one left, and it must go into the fourth shell.
Sometimes people draw the atom out with the nucleus and shells. You will also see the electron structure written as numbers, separated by dots or commas. The numbers start with the first shell, then come in order, so potassium is 2.8.8.1 or 2,8,8,1.
In harder questions, you might be asked about the electron structure for ions. Ions are atoms which have gained or lost electrons. Because electrons are negatively charged, a positive ion has lost electrons, and a negative ion has gained them. For example, a calcium atom has 20 electrons, but a calcium 2+ ion has 18. Once you know how many electrons the ion has, you fill up shells in the same way as for atoms - so Ca2+ is 2.8.8.
It took a while for scientists to work out these rules, but they became very useful. It turned out that a lot of patterns in how different elements behave and react depend on how many electrons there are in the outermost shell. For example, lithium, sodium and potassium all have 1 electron in their outermost shells, and they all have similar properties. The next few activities will help you to understand this idea even better.
Are you ready to have a go at some questions?








