Chlorine is used to make water safe to drink, but was also the original chemical weapon during World War One. Chlorine is one of the halogen elements in Group 7 of the periodic table.
The main Group 7 elements are fluorine, chlorine, bromine and iodine. Together, this group of elements is called the halogens. They are all in the same column of the periodic table, one column in from the right hand side. This means that they are non-metals, and they all form small covalent molecules with two atoms - fluorine is F2, chlorine is Cl2, bromine is Br2, iodine is I2.
All the halogens are very reactive - this is why they are good for killing microbes. Chlorine is used to purify water, and iodine is used to clean wounds, stopping them from becoming infected. Fluorine is the most reactive halogen, then the reactivity decreases as you go down Group 7.
Elements go in the same group of the periodic table because they have the same number of electrons in their outermost electron shell. For halogens, there are seven electrons in the outermost shell, so all halogens need one more electron to have a stable outermost shell. Chlorine (atomic number 17) is 2.8.7, so it looks like this:
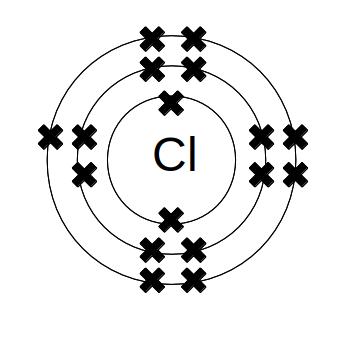
Fluorine is 2.7 and bromine is 2.8.18.7. All the halogens have seven electrons in their outermost shells, and one empty site. The single empty site explains why the halogens are so reactive - they are very close to having a stable outer shell, so will react strongly with other elements in order to capture another electron.
Halogen reactions
All the halogen elements react with other elements in similar ways. There are two reactions you need to know:
alkali metal + halogen → alkali halide
For example, sodium + chlorine → sodium chloride (chlorine becomes chloride)
In symbols, 2Na (s) + Cl2 (g) → 2NaCl (s)
We can replace sodium with any other alkali metal, and chlorine with any other halogen, and this reaction pattern will work in the same way, so
2Cs (s) + Br2 (l) → 2CsBr (s) (bromine is liquid at room temperature, which is why the state symbol is different.)
hydrogen + halogen → hydrogen halide
For example, hydrogen + chlorine → hydrogen chloride
In symbols, H2 (g) + Cl2 (g) → 2HCl (g)
The product formed, hydrogen chloride, is a gas, but it will dissolve in water to form hydrochloric acid, which is why the chemical symbol is the same for hydrogen chloride and hydrochloric acid.
Group 7 trends
|
F Fluorine |
|
Cl Chlorine |
|
Br Bromine |
|
I Iodine |
|
At Astatine |
As you go down Group 7 (from fluorine to astatine):
The elements become less reactive
The melting point and boiling point increase (so they are less likely to be a gas at room temperature)
The colour becomes darker
Testing reactivity with displacement reactions
We can show which halogens are more reactive by looking for displacement reactions between them. The rule is the same one as for metal displacement reactions:
The more reactive element can displace the less reactive element from a compound.
The experiment works like this:
Start with an alkali halide compound dissolved in water - for example, potassium bromide.
Add another halogen, either dissolved in water, or as bubbles of gas.
Check for any colour changes which would tell us if a reaction had happened.
So if we add chlorine to potassium bromide, we get the reaction below because chlorine is more reactive than bromine:
potassium bromide + chlorine → potassium chloride + bromine, and we see the mixture turn orange (because of the bromine)
If we add iodine to potassium bromide, nothing happens, because iodine is less reactive than bromine.
There's a lot you need to know about halogens. It might seem a bit daunting at first, but start with the electron structure. That explains why they form covalent molecules, and why they are all so reactive.
Try the questions now, to secure your understanding of what's going on.








