All materials - living and non-living - are made of atoms. They combine in millions of different ways to make everything we see around us, even us! Scientists discovered the structure of the atom and this helped them to arrange the elements in order on the periodic table. Mendeleev was the pioneer, arranging the elements in order of relative atomic mass. He realised that the properties of elements were related to their relative atomic mass and put the elements with similar properties into vertical groups, the same groups we see on the periodic table today. However, at the time, not all elements had been discovered, but that did not put him off. He simply realised that there were more elements to be discovered and left gaps on the periodic table for those elements! Mendeleev went even one step further to predict the properties of the missing elements and turned out to be right when those elements were finally discovered! A feat of brilliance that has earned him a spot in every scientist's ‘great scientist’ list.

Dmitri Mendeleev (1834-1907), a Russian chemist, published the periodic table in 1869.
But Mendeleev wasn’t the first person to try and do that and he had help from his predecessors. A guy called Döbereiner in the 1800’s put the elements into groups of threes called the triads. He noticed that they all had similar properties and that when you took an average of their atomic mass, it always turned out to be the same as the middle mass. Well, it happened most of the time…
Then a guy called Newlands came along and noticed that there were patterns with every eight elements when you lined them up by atomic mass. So lithium, sodium and potassium all had similar properties and were all eight spaces away from each other – this was a big deal. But it only worked for the first twenty elements, so something was going on.
Mendeleev worked out that if you put the two groups together and then left gaps, you could make it all work – he had just discovered the periodic table.
In 1913 a British chemist, Henry Moseley, realised that it would be better to use atomic number rather than mass, so he put the final touches to the periodic table, after resolving some issues that had surfaced from Mendeleev's work.
The periodic table, as we know it today, is shown below. Familiarise yourself with the position of alkali metals (Group 1), halogens (Group 7), metals and non-metals, transition metals and also noble gases (Group 0).
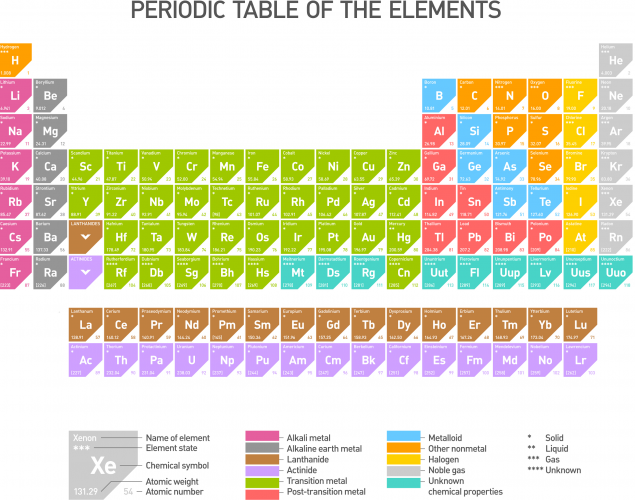
You will need to know this really well for your exam, so spend some time studying it very carefully before moving on to the questions.








