Once you know about protons, neutrons and electrons, you'll be able to work out a lot of the patterns that tell us how chemistry works.
Everything is made of atoms, and atoms are very, very small. They are nearly spherical and their radius is about 1 nanometre. 'Nano' means one billionth, so we can also write this as 0.000 000 001 m, or 10-9 m. The nucleus in the middle of the atom is even smaller, about 1/10 000 of the radius to be exact.
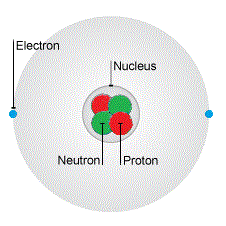
(This picture isn't to scale - the nucleus is much smaller than this!)
All atoms are made of protons, neutrons and electrons - this is what you need to know about them:
| Particle | Relative mass | Relative charge | Where they are found |
| Proton | 1 | +1 | Nucleus |
| Neutron | 1 | 0 | Nucleus |
| Electron | 0.0005 | -1 | Shells |
(Remember: Protons are positive, neutrons are neutral).
If we want to know what an atom is like, we need to know how many protons, neutrons and electrons it has. There are two terms you need to know:
The atomic number of an element is how many protons there are in the nucleus.
The mass number of an element is the total number of protons and neutrons in the nucleus. We don't count electrons because they are much lighter.
When you look up an element in the periodic table, it will tell you the atomic number and mass number, like this:

Different periodic tables put the numbers in different places, so remember that the larger number is the mass number, and the smaller number is the atomic number. It's also a good idea to get a copy of the version of the periodic table your exam board uses, so that you can get used to it.
To work out the numbers of protons, neutrons and electrons, follow these rules:
The number of protons is the same as the atomic number:
So sodium has 11 protons
The number of neutrons is the difference between the mass number and the atomic number:
So the number of neutrons in sodium = 23 - 11 = 12 neutrons
The number of electrons in an atom is the same as the number of protons:
So sodium atoms have 11 electrons
These questions can seem a bit scary to start with, but all you have to do is addition and subtraction. Once you've done some for yourself, it becomes easy to talk about atomic numbers and mass numbers.
Now let's move on to some questions.








